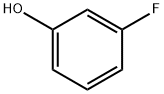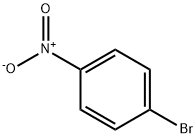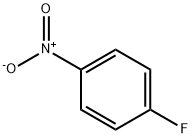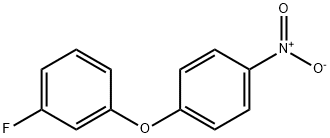
Benzene, 1-fluoro-3-(4-nitrophenoxy)- synthesis
- Product Name:Benzene, 1-fluoro-3-(4-nitrophenoxy)-
- CAS Number:34859-79-5
- Molecular formula:C12H8FNO3
- Molecular Weight:233.2
Yield:34859-79-5 85 %
Reaction Conditions:
with copper(l) iodide;2,2'-bipyridylamine;caesium carbonate in 1,4-dioxane at 110;Inert atmosphere;
Steps:
Preparation of compounds 3a-3t
General procedure: To a 10 mL vial were charged with CuI (19 mg, 0.1 mmol), DPA (34 mg, 0.2 mmol), Cs2CO3 (78 mg, 2.0 mmol), phenols (1.0 mmol) and aryl bromides (1.0 mmol) in 1, 4- dioxane (5 mL). The flask was evacuated and backfilled with argon three times, and the resulting suspension was heated in a 110 C oil bath with rapid stirring for 24 h. After the complete consumption of aryl bromides monitored by TLC, the reaction mixture was cooled to r.t. the flask was opened to air, and the reaction mixture was (if the product was acidic, the mixture was acidified.) diluted with ethyl acetate (5 mL), filtered via a celite pad, and washed with ethyl acetate (10 20 mL). The combined organic phase was concentrated, and the resulting residue was purified by column chromatography on silica gel to provide the desired product.
References:
Chen, Yuanguang;Xu, Huashen;Chen, Lu;Hou, Anyuan;Chen, Guoliang [Synthetic Communications,2022,vol. 52,# 22,p. 2163 - 2170]