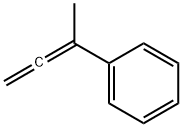
Benzene,(1-methyl-1,2-propadien-1-yl)- synthesis
- Product Name:Benzene,(1-methyl-1,2-propadien-1-yl)-
- CAS Number:22433-39-2
- Molecular formula:C10H10
- Molecular Weight:130.19
Yield:22433-39-2 501 mg
Reaction Conditions:
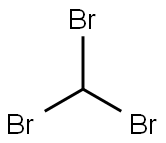
Stage #1: Bromoform;isopropenylbenzenewith N-benzyl-N,N,N-triethylammonium chloride;sodium hydroxide in dichloromethane;water at 20;Inert atmosphere;Sealed tube;Doering-Laflamme Allene Synthesis;
Stage #2: with ethylmagnesium bromide in tetrahydrofuran;diethyl ether at 20; for 1 h;Inert atmosphere;Doering-Laflamme Allene Synthesis;
Steps:
General procedure for the synthesis of allenes
General procedure: The alkene intermediate (300 mg, 1.00 equiv, 1.25 mmol) was added to a 6 dram vial equipped with a stirbar, followed by DCM (4.0 mL), TEBA (3 mg, 0.01 equiv, 10 mol), bromoform (0.14 mL, 1.3 equiv,1.6 mmol), and a 50% w/w solution of aqueous sodium hydroxide (0.170 mL, 5.00 equiv, 6.24 mmol).The vial was purged with argon, capped, and sealed with parafilm. The biphasic solution stirred at roomtemperature for 48 h, at which point the reaction was diluted with 5 mL DI water. The aqueous phase wasextracted with DCM (2 x 10 mL) and dried over sodium sulfate. Solvent was removed under reducedpressure and the crude reaction mixture was purified on silica (10% EtOAc in hexanes) affording thediboromocyclopropyl intermediate as a white solid (304 mg, 0.740 mmol, 59%). The dibromocyclopropyl intermediate (218 mg, 1.00 equiv, 0.529 mmol) was added to a round bottomflask, which was then purged with argon. THF (1.8 mL) was added and the reaction was stirred at roomtemperature. A solution of EtMgBr (0.26 mL, 1.5 equiv, 0.79 mmol, 3 M in diethyl ether) was addeddropwise. The reaction was allowed to stir for 1 h, at which time water (2 mL) was added slowly. Theaqueous layer was extracted with diethyl ether (2 x 10 mL). The organic layer was dried over sodiumsulfate and concentrated in vacuo. The crude reaction mixture was purified via column chromatography(15% EtOAc in hexanes) to afford allene S18 as a white solid (106 mg, 0.420 mmol, 79%).
References:
Gates, Ashley M.;Santos, Webster L. [Synthesis,2019,vol. 51,# 24,p. 4619 - 4624] Location in patent:supporting information

17343-73-6
6 suppliers
$106.00/50MG

22433-39-2
0 suppliers
inquiry
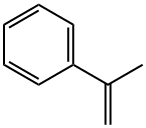
98-83-9
294 suppliers
$10.00/25mL

22433-39-2
0 suppliers
inquiry
1504-58-1
188 suppliers
$8.00/1g

22433-39-2
0 suppliers
inquiry

108-86-1
496 suppliers
$10.00/5g

22433-39-2
0 suppliers
inquiry