
Benzene, 2-chloro-1,5-dimethyl-3-nitro- synthesis
- Product Name:Benzene, 2-chloro-1,5-dimethyl-3-nitro-
- CAS Number:16618-56-7
- Molecular formula:C8H8ClNO2
- Molecular Weight:185.61
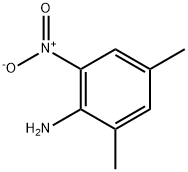
1635-84-3
128 suppliers
$5.00/100mg

16618-56-7
9 suppliers
inquiry
Yield:16618-56-7 81%
Reaction Conditions:
Stage #1: 2,4-Dimethyl-6-nitroanilinwith hydrogenchloride;sodium nitrite in water;acetic acid at 0; for 7.5 h;
Stage #2: with copper(l) cyanide in water;acetic acid at 0 - 20; for 2 h;
Steps:
77
To a solution of 4,6-dimethyl-2-nitroaniline (3 g, 18.07 mmol) in acetic acid (20 mL) and 6 N HCl (60 mL) at 0° C. was added a solution of sodium nitrite (2.18 g, 31.62 mmol) in water (5 mL). The reaction mixture was stirred at 0° C. for 30 min after completion of addition and copper (I) cyanide (3.24 g, 3 mmol) was added pinch by pinch. The resulting mixture was stirred at 0° C. for 5 h and at room temperature for an additional 2 h. The mixture was passed through a Celite pad, extracted with EtOAc (3×100 mL), and concentrated using a rotary evaporator to afford a solid residue. The solid was further purified by column (SiO2, hexanes/EtOAc=7:1) to yield 2-chloro-1,5-dimethyl-3-nitro-benzene (2.6 g, 81%) as a light yellow solid. A solution of 2-chloro-1,5-dimethyl-3-nitro-benzene (2.6 g, 15.7 mmol) and copper (I) cyanide (7.05 g, 78.3 mmol) in DMAC (20 mL) was stirred at reflux for 14 h. The reaction mixture was cooled to room temperature, quenched by adding water (30 mL), filtered through a Celite pad, extracted with EtOAc (3×100 mL), and concentrated using a rotary evaporator to afford a solid residue. The solid was further purified by column (SiO2, hexanes/EtOAc=6:1) to yield 0.64 g of 2,4-dimethyl-6-nitro-benzonitrile (23%). A solution of 2,4-dimethyl-6-nitro-benzonitrile (1.1 g, 6.24 mmol) in MeOH (20 mL) and water (10 mL) was mixed with hydrogen peroxide (10 mL), DMSO (10 mL) and potassium hydroxide (0.636 g, 11.36 mmol). The reaction mixture was stirred at 60° C. for 3 h, diluted with water (100 mL), extracted with EtOAc (3×100 mL), and concentrated using a rotary evaporator to afford 4,6-dimethyl-2-nitrobenzamide (0.52 g, 43%). A solution of 4,6-dimethyl-2-nitrobenzamide (0.52 g, 2.68 mmol) in MeOH (30 mL) was mixed with palladium carbon (0.25 g). The resulting suspension was stirred at room temperature under hydrogen for 14 h. The mixture was passed through a Celite pad, concentrated using a rotary evaporator to afford 2-amino-4,6-dimethyl benzamide (0.42 g, 95%).A mixture of 2-amino-4,6-dimethyl benzamide (0.2 g, 1.22 mmol), 4-[2-(tert-butyl-dimethyl-silanyloxy)-ethoxy]-3,5-dimethyl-benzaldehyde (0.376 g, 1.22 mmol), sodium hydrogensulfite (0.22 g, 1.22 mmol) and prtoluenesulfonic acid (0.116 g, 0.61 mmol) in N,N-dimethyl acetamide (10 mL) was stirred at 155° C. for 14 h. The reaction mixture was cooled to room temperature and diluted with water (50 mL). The solid crashed out and was collected by filtration to afford impure product. The solid was re-dissolved in THF (30 mL) and mixed with TBAF in THF (5 mL, 5 mmol). The reaction mixture was stirred at room temperature for 14 h and concentrated using a rotary evaporator to afford an oily residue. Further purification by column (SiO2, EtOAc/DCM/MeOH=12:4:1) yielded an off-white solid. This solid was diluted with MeOH (10 mL) to make a slurry. The solid was collected by filtration and washed with MeOH to afford 2-(4-(2-hydroxyethoxy)-3,5-dimethylphenyl)-5,7-dimethylquinazolin-4(3H)-one (98 mg, 24%) as a white solid. Selected data: MS (ES) m/z: 339.10; MP 259.6-261.2° C.
References:
US2008/188467,2008,A1 Location in patent:Page/Page column 52-53
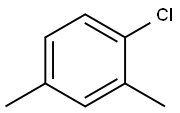
95-66-9
104 suppliers
$49.25/10g

16618-56-7
9 suppliers
inquiry

108-38-3
353 suppliers
$10.60/50gm:

16618-56-7
9 suppliers
inquiry