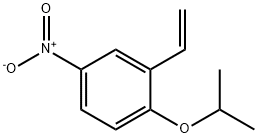
Benzene, 2-ethenyl-1-(1-Methylethoxy)-4-nitro- synthesis
- Product Name:Benzene, 2-ethenyl-1-(1-Methylethoxy)-4-nitro-
- CAS Number:502848-71-7
- Molecular formula:C11H13NO3
- Molecular Weight:207.23
Yield:502848-71-7 63%
Reaction Conditions:
with n-butyllithium in tetrahydrofuran;hexane at -78 - 20; for 10 h;
Steps:
1
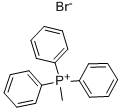
To a stirred suspension of powdered anhydrous potassium carbonate (1.1 g, 8 mmol), acatalytic amount of caesium carbonate (521 mg, 40 mol%) and 3.1 (668 mg, 4 mmol) indry DMF (25 ml) was added neat 2-iodopropane (0.8 ml, 8 mmol). The reacting mixturewas stirred for 24 h at room temperature (RT), and then the solvent was evaporated invacuum. The residue was poured to water (50ml) and extracted with tBuOMe (4x25 ml).The combined organic layers were washed with brine, dried with Mg2SO4 and evaporatedto dryness. Crude product was purified using silica-gel column chromatography(cyclohexane / EtOAc 8:2), to give 2-iso-propoxy-5-nitrobenzaldehyde 4.I as low-meltingsolid (850 mg, 86% of yield).IR (KBr): ν [cm1-] = 3115, 2991, 2942,1679, 1609, 1526, 1348,1284, 1111, 950, 832,748, 667;1H-NMR (500 MHz, CDCl3): δ[ppm]= 1.48 (d, 6H, J = 6.1 Hz), 4.85 (q, 1H, J = 6.1 Hz), 7.10 (d, 1H, J = 9.2 Hz), 8.39 (dd, 1H, J = 2.9, 9.2 Hz), 8.69 (d, 1H, J = 2.9 Hz), 10.41 (s,1H);13C-NMR (125 MHz, CDCl3): δ[ppm] = 21.8, 72.6, 113.6, 124.7, 125.12, 130.4, 141.1, 164.3, 187.8;MS(El): m/z 209 (10, [M]+.), 167 (100), 137 (18), 120 (11), 93 (7), 75 (3), 65 (10), 53 (4); HRMS(El) calculated for [M]+ (C10H11O4N): 209.0688; found 209.0686.202530 2.I To a stirred suspension of Ph3PCH3Br (932 mg, 2.53 mmol) in dry THF (20 ml) was added slowly at -78 °C successive a solution of BuLi in hexane (1.8 ml, 2.7 mmol, 1.5M) and a solution of 4.1 in dry THF (2 ml). After this the reaction mixture was allowed to warm to r.t. and was stirred for additional 10 h. After this time a saturated solution of NH4CI (2 ml) and tBuOMe (100 ml) were added. Insoluble material was filtered-off and the resulting solution evaporated in vacuum. Crude product was purified by column chromatography on silica-gel (using cyclohexane / EtOAc 8:2) to give 2-iso-propoxy-5-nitrostyrene 2.I as pale yellow oil (236 mg, 63% of yield).IR (film): ν [cm-1] = 3088, 2982, 2967,1627,1607, 1583,1516,1341, 1271,1107, 950, 742 cm-1; 1H-NMR (500 MHz, CDCl3): δ[ppm] = 1.41 (d, 6H, J = 6.0 Hz), 4.71 (q, 1H, J = 6.0 Hz), 5.40 (dd, 1H, J = 0.5,11.2 Hz), 5.87 (dd, 1H, J = 0.5,17.7 Hz), 6.91 (d, 1H, J = 9.1 Hz), 7.00 (dd, 1H, J = 11.2, 17.7 Hz), 8.12 (dd, 1H, J = 2.8, 9.1 Hz), 8.36 (d, 1H, J = 2.8 Hz); 13C-NMR (125 MHz, CDCl3): δ[ppm] = 21.9, 71.5,112.2,116.8,122.4,124.5, 128.1,130.1,141.0,159.9; MS (El): m/z 207 (4, [M]+.), 165 (59), 148 (100), 135 (4), 118 (96), 104 (2), 90 (15), 65 (8), 63 (7), 51 (4); MS (ESI): m/z 230 ([M+Na]+); HRMS (ESI): m/z calculated for [M+Na]+ (C11H13O3NNa): 230.0788; found 230.0776.
References:
WO2004/35596,2004,A1 Location in patent:Page/Page column 12-13

97-51-8
414 suppliers
$6.00/5g

502848-71-7
0 suppliers
inquiry

90-02-8
444 suppliers
$9.00/1g

502848-71-7
0 suppliers
inquiry
22921-58-0
72 suppliers
$42.00/500mg

502848-71-7
0 suppliers
inquiry