
Benzeneacetaldehyde,2,5-dimethoxy- synthesis
- Product Name:Benzeneacetaldehyde,2,5-dimethoxy-
- CAS Number:33567-62-3
- Molecular formula:C10H12O3
- Molecular Weight:180.2

7417-19-8
25 suppliers
$489.00/1g

33567-62-3
12 suppliers
inquiry
Yield:33567-62-3 83%
Reaction Conditions:
with Dess-Martin periodane in dichloromethane at 20; for 5 h;Inert atmosphere;
Steps:
To a flame-dried round bottom flask was charged Dess-Martin periodinane (2.51 g, 5.93 mmol), which was dissolved with DCM (25 mL). The resulting solution was allowed to stir for several minutes at room temperature. Finally, 2-(2,5-dimethoxyphenyl)ethan-1-ol 8 (900 mg, 4.94 mmol) was added dropwise by syringe. The reaction was allowed to proceed at room temperature for 5 hours. The reaction mixture was quenched with a 1:1 mixture of sat. aq. NaHCO3 and sat. aq. NaS2O3 (20 mL). The resulting layers were separated, and aqueous layer was extracted with DCM (3 x 20 mL). The combined organic phase was washed with brine, dried over MgSO4, and concentrated under reduced pressure. Purification by silica gel chromatography (100% hexanes to 1:1 hexanes:EtOAc eluent, 15 min) provided the desired product (2-(2,5-dimethoxyphenyl)acetaldehyde 5 (0.74 g, 83 %)) as a translucent oil. 1HNMR and 13CNMR spectra were consistent with those reported in the literature.
References:
Kim, Dalton;Nash, Aaron;De Brabander, Jef;Tambar, Uttam K. [Tetrahedron,2018,vol. 74,# 28,p. 3787 - 3790] Location in patent:supporting information
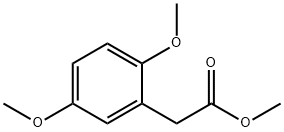
6202-39-7
1 suppliers
inquiry

33567-62-3
12 suppliers
inquiry
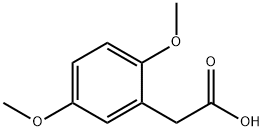
1758-25-4
194 suppliers
$27.50/5g

33567-62-3
12 suppliers
inquiry
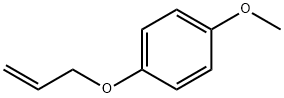
13391-35-0
30 suppliers
$40.00/5mg

33567-62-3
12 suppliers
inquiry
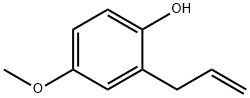
584-82-7
20 suppliers
$50.00/25mg

33567-62-3
12 suppliers
inquiry