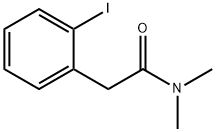
Benzeneacetamide, 2-iodo-N,N-dimethyl- synthesis
- Product Name:Benzeneacetamide, 2-iodo-N,N-dimethyl-
- CAS Number:75117-26-9
- Molecular formula:C10H12INO
- Molecular Weight:289.11
1H NMR (CDCl3): δ 7.83 (dd, J ) 8.1, 1.3 Hz, 1H), 7.31 (ddd, J ) 7.5, 7.4, 0.8 Hz, 1H), 7.26 (dd, J ) 7.5, 1.7 Hz, 1H), 6.94 (ddd, J ) 7.5, 7.4, 1.7 Hz, 1H), 3.80 (s, 2H), 3.04 (s, 3H), 3.01 (s, 3H). 13C NMR (CDCl3): δ 170.13, 139.32, 139.29, 138.72, 129.85, 128.48, 101.09, 45.77, 37.66, 35.65.
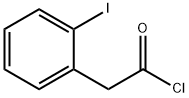
62300-07-6
11 suppliers
$116.64/250mgs:

124-40-3
498 suppliers
$18.00/100ml

75117-26-9
11 suppliers
inquiry
Yield:75117-26-9 99%
Reaction Conditions:
in dichloromethane at 0;
References:
Lachia, Mathilde;Jung, Pierre M.J.;De Mesmaeker, Alain [Tetrahedron Letters,2012,vol. 53,# 34,p. 4514 - 4517] Location in patent:scheme or table
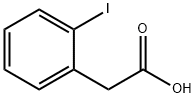
18698-96-9
237 suppliers
$5.00/250mg

124-40-3
498 suppliers
$18.00/100ml

75117-26-9
11 suppliers
inquiry

18698-96-9
237 suppliers
$5.00/250mg
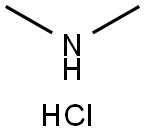
506-59-2
528 suppliers
$5.00/5G

75117-26-9
11 suppliers
inquiry

18698-96-9
237 suppliers
$5.00/250mg

75117-26-9
11 suppliers
inquiry