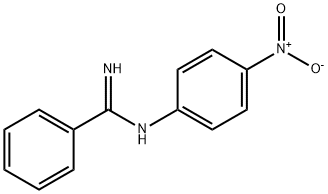
BENZENECARBOXIMIDAMIDE,N-(4-NITROPHENYL)- synthesis
- Product Name:BENZENECARBOXIMIDAMIDE,N-(4-NITROPHENYL)-
- CAS Number:1986-61-4
- Molecular formula:C13H11N3O2
- Molecular Weight:241.25
Yield:-
Reaction Conditions:
with aluminum (III) chloride at 0 - 140; for 2 h;Inert atmosphere;Sealed tube;
Steps:
2. Preparation of Amidine
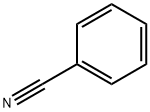
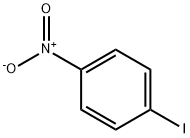
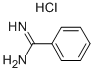
General procedure: A mixture of the carbonitrile (10.0 mmol, 1.0 equiv) and the aniline (11.0 mmol, 1.1 equiv) were weighted in a sealed tube equipped with a stir bar, and AlCl3 (10.0 mmol, 1.0 equiv) was added portionwise under 0 °C. Then the mixture was stirred at 140 °C for 2.0 h under argon atmosphere. After completion, the reaction mixture was extracted with DCM, and poured into a concentrated NaOH solution (100 mL) under 0 °C. Then the resulting mixture stirred for about 15 min and was extracted with DCM. The combined organic layers were washed with brine (30 mL × 3), and dried overanhydrous Na2SO4, and evaporated under vacuum. The residue was purified either by silica gel chromatography (solvent DCM/ Methanol) to afford the derivatives
References:
Huang, Xin;Xu, Yingying;Li, Jianglian;Lai, Ruizhi;Luo, Yi;Wang, Qiantao;Yang, Zhongzhen;Wu, Yong [Chinese Chemical Letters,2021,vol. 32,# 11,p. 3518 - 3521] Location in patent:supporting information
![Benzenecarboximidamide, N-[(4-nitrophenyl)sulfonyl]-](/CAS/20210305/GIF/30924-69-7.gif)
30924-69-7
0 suppliers
inquiry

1986-61-4
0 suppliers
inquiry