
Benzeneethanol, β-methylene-α-(1-methylethyl)- synthesis
- Product Name:Benzeneethanol, β-methylene-α-(1-methylethyl)-
- CAS Number:140226-81-9
- Molecular formula:C12H16O
- Molecular Weight:176.25
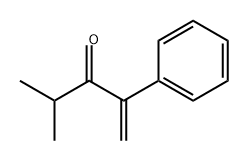
67516-83-0
0 suppliers
inquiry

140226-81-9
0 suppliers
inquiry
Yield:-
Reaction Conditions:
with sodium tetrahydroborate;cerium(III) chloride heptahydrate in methanol at 0; for 0.25 h;
Steps:
4.3. The preparation of allylic alcohols10,11
General procedure: To a stirred solution of phenyl-2-propanone (10 equiv) in DMF was successively added paraformaldehyde (50 equiv), piperidine (1.3 equiv) and AcOH (2.2 equiv). The resulting mixture was heating at 90 °C for 1 h. After cooling, water was added to the residue and the mixture was extracted with EA. The combined organic layers were washed with water, dried over MgSO4, filtered, and concentrated to give in vacuo vinyl ketone. To a solution of vinyl ketone (1 equiv) in methanol at 0 °C were added cerium trichloride (1 equiv) and sodium borohydride (1 equiv). The reaction mixture was stirred for 15 min at 0 °C and then saturated aqueous sodium hydrogen carbonate was added. The mixture was extracted with diethyl ether, and the organic layer was washed with brine, dried over sodium sulfate. After filtration of the mixture and evaporation of the solvent, the crude product was purified by column chromatography to afford the allylic alcohols 1.
References:
Jiang, Shan-Shan;Gu, Bo-Qi;Zhu, Ming-Yu;Yu, Xingxin;Deng, Wei-Ping [Tetrahedron,2015,vol. 71,# 8,p. 1187 - 1191]