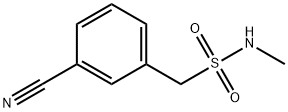
Benzenemethanesulfonamide, 3-cyano-N-methyl- synthesis
- Product Name:Benzenemethanesulfonamide, 3-cyano-N-methyl-
- CAS Number:933989-11-8
- Molecular formula:C9H10N2O2S
- Molecular Weight:210.25
Yield:933989-11-8 69%
Reaction Conditions:
Stage #1: m-(bromomethyl)benzonitrilewith sodium sulfite in ethanol;water; for 4 h;Heating / reflux;
Stage #2: with oxalyl dichloride;N,N-dimethyl-formamide in dichloromethane at 0 - 20; for 3 h;
Stage #3: methylamine in tetrahydrofuran; for 12 h;
Steps:
70.1
Example 70; 2-(3-Fluoro-phenylV4-methyl-pyrimidine-5-carboxylic acid 3-methylsulfamoylmethyl-benzylamide; Step 1; A solution of 3-bromomethyl-benzonitrile (2 g, 10.2 mmol) in EtOH (50 mL) is treated with a solution of Na2SOs (1.3 g, 10.3 mmol) in H2O (50 mL) and heated at reflux for 4 hours. The mixture is concentrated in vacuo. The residue is suspended in DCM (100 mL) and DMF (1 mL), cooled to O0C and treated with oxalyl chloride (8 mL, 43.4 mmol). The mixture is warmed to room temperature and stirred for 3 hours. The mixture is diluted with brine, extracted with DCM (2x100 mL), dried with Na2SO^ filtered, and concentrated in vacuo. The residue is dissolved in THF (25 mL), methylamine (2 EPO
References:
WO2007/41634,2007,A1 Location in patent:Page/Page column 62-63

