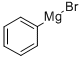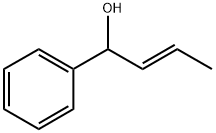
Benzenemethanol, α-(1E)-1-propen-1-yl- synthesis
- Product Name:Benzenemethanol, α-(1E)-1-propen-1-yl-
- CAS Number:52755-39-2
- Molecular formula:C10H12O
- Molecular Weight:148.21
Yield:52755-39-2 99.9%
Reaction Conditions:
in tetrahydrofuran;diethyl ether at 0 - 20; for 7.5 h;
Steps:
Examples; Synthesis of 5-phenyl-2, 4-PENTADIENAL
To a cooled (0-5 °C) 927 mL of 1 M solution of phenyl magnesium bromide in tetrahydrofuran was added dropwise a solution of crotonaldehyde (65.0 g) in 130 mL of anhydrous ether over a period of 2 hours and 45 minutes. The reaction was stirred for an additional 45 minutes and then warmed to room temperature. After four more hours of stirring, saturated ammonium chloride aqueous solution (750 mL) was added to the reaction. The mixture was extracted with 750 mL of ether twice. The combined extract was dried over anhydrous potassium carbonate and filtered. The solvent was evaporated to give 135.88 g (99.9%) of the desired 1-PHENYL-2-BUTEN-1-OL as an oil which was used in the next step without further purification.
References:
WO2003/99272,2003,A1 Location in patent:Page 14-16
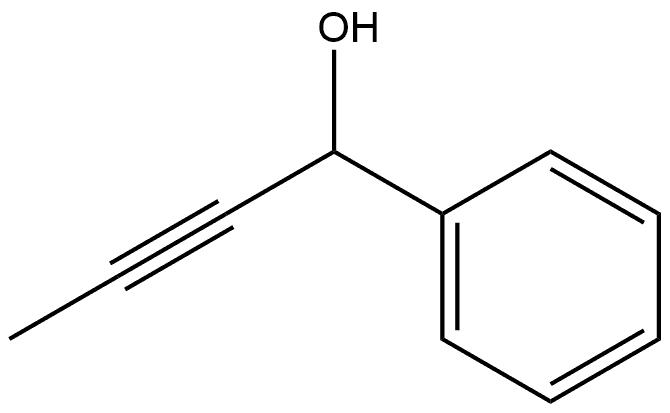
32398-66-6
1 suppliers
inquiry

52755-39-2
1 suppliers
inquiry