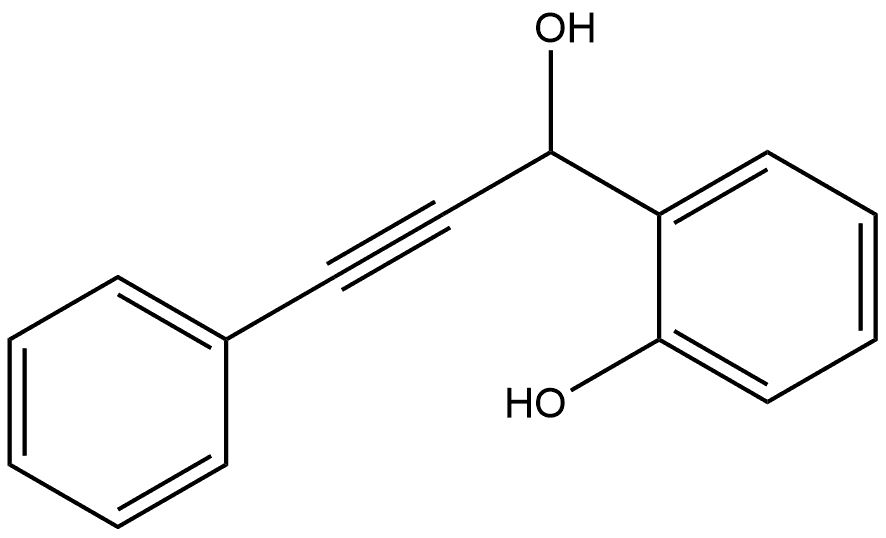
Benzenemethanol, 2-hydroxy-α-(2-phenylethynyl)- synthesis
- Product Name:Benzenemethanol, 2-hydroxy-α-(2-phenylethynyl)-
- CAS Number:109365-86-8
- Molecular formula:C15H12O2
- Molecular Weight:224.25
Yield:109365-86-8 78%
Reaction Conditions:
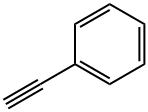
Stage #1: phenylacetylenewith n-butyllithium in tetrahydrofuran;hexane at -40; for 0.5 h;Inert atmosphere;
Stage #2: salicylaldehyde in tetrahydrofuran;hexane at -40 - 0; for 2 h;Inert atmosphere;
Steps:
1) General procedure for synthesis of intermediate.
General procedure: To a solution of arylacetylene (1.6 mmol) in THF (4.0 mL) was dropwise added n-BuLi (1.5 mmol, 2.5 M in n-hexane) at -40 °C under nitrogen atmosphere. After 0.5 h stirring, a solution of corresponding salicylaldehyde (1.0 mmol in 4.0 mL anhydrous THF) was dropwise added at -40 °C The reaction mixture was stirred at -40 °C for 2 h and then heated up to 0 °C for 5 min followed by quenched with water. The organic solvent was removed by rotary evaporator and the rest was extracted with EtOAc twice. The collected EtOAc layer was washed with brine, dried over Na2SO4, filtered, and evaporated. The crude product was purified by flash column chromatography.
References:
Li, Siyuan;Jin, Feng;Viji, Mayavan;Jo, Hyeju;Sim, Jaeuk;Kim, Hak Sung;Lee, Heesoon;Jung, Jae-Kyung [Tetrahedron Letters,2017,vol. 58,# 14,p. 1417 - 1420] Location in patent:supporting information

6738-06-3
15 suppliers
$141.00/50mL
89-95-2
205 suppliers
$22.00/10g

109365-86-8
0 suppliers
inquiry

108-95-2
723 suppliers
$14.00/25g

109365-86-8
0 suppliers
inquiry