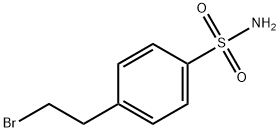
Benzenesulfonamide, 4-(2-bromoethyl)- synthesis
- Product Name:Benzenesulfonamide, 4-(2-bromoethyl)-
- CAS Number:5378-84-7
- Molecular formula:C8H10BrNO2S
- Molecular Weight:264.14
Yield:5378-84-7 264 g (1 mol, 93% based on phenyl ethyl bromide)
Reaction Conditions:
with chlorosulfonic acid;sodium hydroxide in ammonium hydroxide;toluene;
Steps:
I EXAMPLE I
EXAMPLE I This example illustrates the preparation of N-mesyl-4-vinylbenzenesulfonamide. A 2-liter multi-necked round bottom flask fitted with an mechanical stirrer (equipped with a glass stir rod and teflon paddle), reflux condenser, pressure equalizing addition funnel and thermometer was maintained under a sweep of argon. The system was vented to a trap containing 50% sodium hydroxide solution. The flask was charged with 626.4 g (5.4 mol) of chlorosulfonic acid, to this was added, dropwise, 200 g (1.08 mol) of phenyl ethyl bromide at such a rate as to maintain the reaction temperature below 27° C. with external ice-bath cooling. Upon complete addition, the mixture was stirred an additional hour and then cautiously poured over a large excess of ice to precipitate the crude sulfonyl chloride. Most of the ice water was decanted and the product dissolved in approximately one-liter of toluene. This toluene solution of 4-(2-bromethyl) benzenesulfonyl chloride was dried over anhydrous magnesium sulfate, filtered and used without further purification. A 3-liter multi-necked round bottomed flask equipped as above was also maintained under a positive atmosphere of argon. The flask was charged with the toluene solution of 4-(2-bromoethyl) benzenesulfonyl chloride, cooled in an ice bath and 200 ml of 58% ammonium hydroxide introduced dropwise. Upon complete addition, the mixture was brought to between 50°-60° C. for 1.5 hrs. The mixture was cooled to room temperature and the crude sulfonamide collected and dried on a Buchner funnel to yield 264 g (1 mol, 93% based on phenyl ethyl bromide) of 4-(2-bromoethyl) benzenesulfonamide which was used without further purification.
References:
US4510324,1985,A
64062-91-5
2 suppliers
inquiry

5378-84-7
1 suppliers
inquiry
103-63-9
493 suppliers
$9.00/10g

5378-84-7
1 suppliers
inquiry