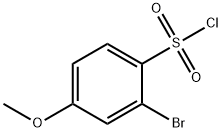
Benzenesulfonyl chloride, 2-broMo-4-Methoxy- synthesis
- Product Name:Benzenesulfonyl chloride, 2-broMo-4-Methoxy-
- CAS Number:23095-16-1
- Molecular formula:C7H6BrClO3S
- Molecular Weight:285.54
2398-37-0
581 suppliers
$8.00/10g

23095-16-1
13 suppliers
inquiry
Yield:23095-16-1 14%
Reaction Conditions:
with chlorosulfonic acid at 0; for 2 h;
Steps:
2-Bromo-4-methoxybenzenesulfonyl chloride S3
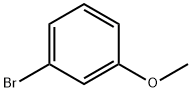
At 0 °C, chlorosulfonic acid (6.2 mL, 0.09mol, 1.8 equiv.) was treated with 3-bromoanisole (9.30 g, 0.05 mol, 1 equiv.) with stirring atsuch a rate that the internal temperature remained below 5 °C. The mixture was stirred at 0°C for 2 h. Following this the mixture was added dropwise to crushed ice (100 g) and wasstirred for a further 30 min. The mixture was extracted with ethyl acetate (3 x 50 mL) andthe combined organic layers were washed with brine (20 mL) and water (20 mL), dried over MgSO4 and concentrated in vacuo. The residue was purified by chromatography on silica gel(c-Hex-EtOAc; 9:1) to afford the compound S3 (1.90 g, 14%) as a light yellow solid
References:
Khalifa, Aisha;Conway, Lorna;Geoghegan, Kimberly;Evans, Paul [Tetrahedron Letters,2017,vol. 58,# 48,p. 4559 - 4562] Location in patent:supporting information

2398-37-0
581 suppliers
$8.00/10g

145915-29-3
29 suppliers
$65.00/10mg

23095-16-1
13 suppliers
inquiry