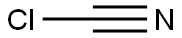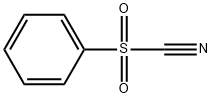
BENZENESULFONYL CYANIDE synthesis
- Product Name:BENZENESULFONYL CYANIDE
- CAS Number:24224-99-5
- Molecular formula:C7H5NO2S
- Molecular Weight:167.19
Yield: 93.8%
Reaction Conditions:
in dichloromethane;water
Steps:
R.1
Reference Example 1
References:
Kuraray Co., Ltd. US6232473, 2001, B1
542-92-7
91 suppliers
inquiry

24224-99-5
9 suppliers
inquiry
5285-87-0
9 suppliers
inquiry

24224-99-5
9 suppliers
inquiry