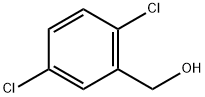
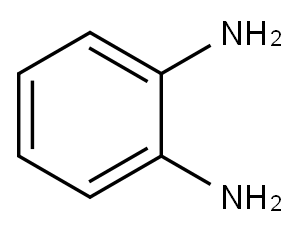
BENZIMIDAZOLE, 2-(2,5-DICHLOROPHENYL)- synthesis
- Product Name:BENZIMIDAZOLE, 2-(2,5-DICHLOROPHENYL)-
- CAS Number:14225-80-0
- Molecular formula:C13H8Cl2N2
- Molecular Weight:263.12
Yield:14225-80-0 92%
Reaction Conditions:
with dimethyl sulfoxide;2,4,6-tripropyl-1,3,5,2,4,6-trioxatriphosphinane-2,4,6-trioxide in ethyl acetate at 0 - 25; for 2.5 h;Inert atmosphere;chemoselective reaction;
Steps:
General procedure for the one-pot synthesis of benzimidazoles and benzothiazoles:
General procedure: to a solution of alcohol (1.1 mmol) in a mixture of solvents ethyl acetate (4 mL) and DMSO (2 mL), was added T3P (2 mmol, 50% solution in ethyl acetate) at 0 °C and the resulting reaction mixture was stirred at room temperature for 1-2 h under nitrogen atmosphere. The reaction was monitored by TLC, 1,2-phenylenediamine (1 mmol) was added and stirred further for 1-2 h. After completion of the reaction, the mixture was diluted with water (20 mL) and neutralized with 10% NaHCO3 solution. The product was extracted with ethyl acetate (10 mL) and the combined organic phase was washed with water (10 mL) and brine solution. The organic phase was dried over anhydrous Na2SO4. The solvent was dried under reduced pressure to afford a crude product, which was purified on silica gel using ethyl acetate and petroleum ether. For the conversion of alcohols to benzothiazoles the same procedure as above was followed except the use of o-aminothiophenol, instead of 1,2-phenylenediamine.
References:
Raghavendra, Goravanahalli M.;Ramesha, Ajjahalli B.;Revanna, Cigalli N.;Nandeesh, Kebbahalli N.;Mantelingu, Kempegowda;Rangappa, Kanchugarakoppal S. [Tetrahedron Letters,2011,vol. 52,# 43,p. 5571 - 5574] Location in patent:experimental part
2905-61-5
179 suppliers
$15.00/250mg

14225-80-0
4 suppliers
inquiry
615-43-0
413 suppliers
$5.00/5g

14225-80-0
4 suppliers
inquiry