
Benzo[b]thiophene-2,3-dione, 6-Methoxy- synthesis
- Product Name:Benzo[b]thiophene-2,3-dione, 6-Methoxy-
- CAS Number:63675-77-4
- Molecular formula:C9H6O3S
- Molecular Weight:194.21

79-37-8
464 suppliers
$17.67/10gm:
15570-12-4
237 suppliers
$17.00/1g
![Benzo[b]thiophene-2,3-dione, 6-Methoxy-](/CAS/GIF/63675-77-4.gif)
63675-77-4
8 suppliers
inquiry
Yield:63675-77-4 47%
Reaction Conditions:
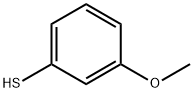
Stage #1: oxalyl dichloride;3-methoxybenzenethiol in diethyl ether at 20; for 1.5 h;Heating / reflux;
Stage #2: with aluminum (III) chloride in dichloromethane at 0 - 20; for 0.5 h;Heating / reflux;
Steps:
I.1
To a solution of 3-methoxythiophenol (26.7 mmol) in ether (20 mL) was added oxalyl chloride (43 mmol) dropwise. The mixture was heated at reflux for 1.5 h, cooled to rt, and concentrated in vacuo. The resulting yellow oil was dissolved in dichloromethane (50 mL), cooled to 0 °C, and was treated with aluminum chloride (32.0 mmol) in portions. The mixture was heated at reflux for 30 min, cooled to rt, and poured onto ice water with stirring. The organic layer was separated and successively washed with saturated, aqueous sodium bicarbonate, water, and brine. The organic layer was dried over magnesium sulfate, filtered and concentrated in vacuo. The residue was purified by chromatography (4/1 ethyl acetate/hexane) which provided 6-methoxy-l- benzothiophene-2,3-dione (47%) as an orange solid. To a mixture of the dione (0.44 mmol) in 30% aqueous solution of ammonium hydroxide (2.0 mL) was added 35% aqueous solution hydrogen peroxide (0.2 mL) and the reaction mixture was maintained for 12 h. The precipitated pink solids were isolated by filtration, washed with water, and dried under high vacuum to afford 6- methoxybenzisothiazole-3-carboxamide (42%). To a solution of the amide (5.46 mmol) in methanol (100 mL) was added 10 N sodium hydroxide (12 mL). The mixture was heated at reflux for 12 h, cooled to rt, and was acidified to pH < 2 by the slow addition of conc. hydrochloric acid. The organic layer was extracted with dichloromethane (2 x) and was dried over sodium sulfate. The crude product was purified by chromatography (300/50/1 dichloromethane/methanol/formic acid) to provide 6-methoxy-1,2-benzisothiazole-3- carboxylic acid (89%) as' a pink solid. The acid was coupled with 1,4- diazabicyclo [3.2.2]nonane according to procedure A.
References:
WO2005/111038,2005,A2 Location in patent:Page/Page column 61-62