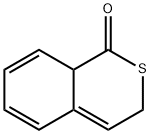
Benzo[c]thiophen-1(3H)-one synthesis
- Product Name:Benzo[c]thiophen-1(3H)-one
- CAS Number:1194-57-6
- Molecular formula:C8H6OS
- Molecular Weight:150.2
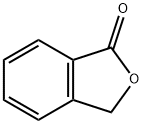
87-41-2
458 suppliers
$6.00/10g
![Benzo[c]thiophen-1(3H)-one](/CAS/GIF/1194-57-6.gif)
1194-57-6
7 suppliers
inquiry
Yield: 18%
Reaction Conditions:
with indium(III) triflate;hexamethyldisilathiane in 1,2-dichloro-benzene at 80; for 168 h;Sealed tube;Glovebox;
Steps:
3.2. General Procedure A for the Indium-Catalyzed Conversion of Lactones or Their Derivatives 1 intoThiolactones 2 Using a Disilathiane (In the Case of 1 in Solid State at Room Temperature)
General procedure: To a screw-capped tube, lactone or the derivative 1 (0.50 mmol) was added. The tube was sealedand moved into a glovebox, then In(OTf)3 (2.8 mg, 0.0050 mmol) was added. The tube was sealedagain and removed from the glovebox. 1,2-Dichlorobenzene (0.5 mL) and hexamethyldisilathiane(98.1 mg, 0.550 mmol) were successively added, and after the tube was sealed, the mixture was heatedat 80 °C for 24 h. The resulting mixture was cooled to room temperature and chloroform was added.The mixture was transferred into a round-bottom flask, which was then evaporated under reducedpressure. The crude material was purified by silica gel column chromatography (hexane/EtOAc)followed by gel permeation chromatography (GPC) in some cases.
References:
Ogiwara, Yohei;Takano, Ken;Horikawa, Shuhei;Sakai, Norio [Molecules,2018,vol. 23,# 6,art. no. 1339]
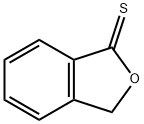
4741-68-8
0 suppliers
inquiry
![Benzo[c]thiophen-1(3H)-one](/CAS/GIF/1194-57-6.gif)
1194-57-6
7 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![Benzo[c]thiophen-1(3H)-one](/CAS/GIF/1194-57-6.gif)
1194-57-6
7 suppliers
inquiry
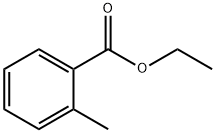
87-24-1
146 suppliers
$14.00/1g
![Benzo[c]thiophen-1(3H)-one](/CAS/GIF/1194-57-6.gif)
1194-57-6
7 suppliers
inquiry
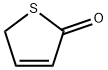
3354-32-3
25 suppliers
$97.79/1g
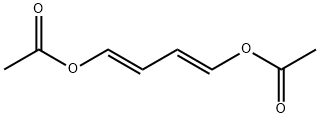
15910-11-9
15 suppliers
$147.00/1g
![Benzo[c]thiophen-1(3H)-one](/CAS/GIF/1194-57-6.gif)
1194-57-6
7 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry