
Benzofuran, 3-bromo-2-(bromomethyl)- synthesis
- Product Name:Benzofuran, 3-bromo-2-(bromomethyl)-
- CAS Number:447402-12-2
- Molecular formula:C9H6Br2O
- Molecular Weight:289.95
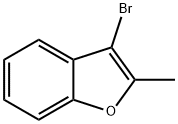
58863-48-2
29 suppliers
$99.00/100mg

447402-12-2
0 suppliers
inquiry
Yield:447402-12-2 79%
Reaction Conditions:
with N-Bromosuccinimide;2,2'-azobis(isobutyronitrile) in chlorobenzene at 80; for 0.5 h;
Steps:
60 Production of 3-bromo-2-bromomethylbenzofuran
2.7 g (15.3 mmoles) of N-bromosuccinimide and 0.4 g (2.7 mmoles) of azobisisobutyronitrile were added to a solution of 2.8 g (13.3 mmoles) of 3-bromo-2-mehtylbenzofuran dissolved in 30 ml of monochlorobenzene. The mixture was stirred at 80°C for 30 minutes to give rise to a reaction. After confirmation of the disappearance of the raw materials, the reaction mixture was cooled to room temperature. The insolubles were removed by filtration. The filtrate was subjected to vacuum distillation to remove the solvent contained therein. The residue was poured into water, followed by extraction with ethyl acetate. The resulting organic layer was washed with water and an aqueous sodium chloride solution in this order and then dried over anhydrous magnesium sulfate. The resulting solution was subjected to vacuum distillation to remove the solvent contained therein, to obtain 3.0 g (yield: 79.0%) of 3-bromo-2-bromomethylbenzofuran.
References:
EP1364946,2003,A1 Location in patent:Page/Page column 204