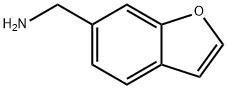
benzofuran-6-ylMethanaMine synthesis
- Product Name:benzofuran-6-ylMethanaMine
- CAS Number:17450-69-0
- Molecular formula:C9H9NO
- Molecular Weight:147.17
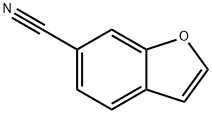
17450-68-9
32 suppliers
inquiry

17450-69-0
15 suppliers
inquiry
Yield:17450-69-0 48.6%
Reaction Conditions:
with lithium aluminium hydride in tetrahydrofuran at 20; for 1 h;
Steps:
Benzofuran-6-ylmethanamine (14)
To a mixture of LiAlH4 (265 mg, 6.99 mmol) in THF(10.0 mL), compound 13 (1.00 g, 6.99 mmol) was added inone portion at 20 °C. The mixture was stirred at 20 °C for1 h. TLC indicated one new spot was detected. 1 mL ofsaturated Na2SO4 was added to the reaction mixture carefully,and then filtered. The filtration was concentrated togive compound 14 (0.5 g, 3.40 mmol, 48.60% yield) as alight yellow oil. 1H NMR (MeOD, 400 MHz) δ 7.71(d, J = 2.0 Hz, 1H, H-2), 7.55 (d, J = 8.0 Hz, 1H, H-4), 7.49(s, 1H, H-7), 7.21 (d, J = 8.0 Hz, 1H, H-5), 6.79-6.80 (m,1H, H-3), 3.88 (s, 2H, CH2, CH2NH2); LCMS m/z 148.1[M + H]+.
References:
Du, Guoxin;Du, Weiwei;An, Yuanlong;Wang, Minnan;Hao, Feifei;Tong, Xiaochu;Gong, Qi;He, Xiangdong;Jiang, Hualiang;He, Wei;Zheng, Mingyue;Zhang, Donglei [Medicinal Chemistry Research,2022,vol. 31,# 4,p. 555 - 579]
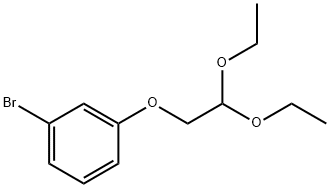
204452-94-8
6 suppliers
inquiry

17450-69-0
15 suppliers
inquiry

591-20-8
350 suppliers
$10.00/1g

17450-69-0
15 suppliers
inquiry
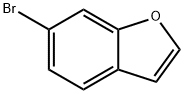
128851-73-0
125 suppliers
$23.00/100mg

17450-69-0
15 suppliers
inquiry