
BENZOIC ACID, 2,4-DIHYDROXY-6-PENTYL-, ETHYL ESTER synthesis
- Product Name:BENZOIC ACID, 2,4-DIHYDROXY-6-PENTYL-, ETHYL ESTER
- CAS Number:38862-65-6
- Molecular formula:C14H20O4
- Molecular Weight:252.31

58497-40-8
1 suppliers
inquiry

38862-65-6
94 suppliers
$19.00/250mg
Yield:-
Reaction Conditions:
with palladium 10% on activated carbon;hydrogen;sodium citrate in ethanol;water at 55 - 65; under 3800.26 Torr; for 14 h;Autoclave;
Steps:
4 Preparation of 2,4-Dihydroxy-6-n-pentyl-benzoic acid ethyl ester (Compound 3)
Example 4
Preparation of 2,4-Dihydroxy-6-n-pentyl-benzoic acid ethyl ester (Compound 3)
To a 2 L Parr stirring pressure vessel was added compound 2 (75.0 g, 0.18 mol, 1.0 eq), sodium citrate (98.4 g, 0.46 mol, 2.6 eq), 10% palladium on carbon (wet 50% water, 11.7 g), water (210 mL), ethanol (140 mL).
The vessel was sealed and flushed with nitrogen and subsequently was pressurized with hydrogen (5 atm) with rapid stirring as stirring rate affects the reaction rate.
The solution was heated to 55° C.-65° C. and it was allowed to stir at this temperature for 14 h.
The solution was sampled and the reaction was complete by HPLC.
The reactor was then cooled to room temperature and was flushed with nitrogen.
The solution was filtered through a 75 g celite pad and the pad was washed with 150 mL of a (1:1) toluene water solution.
The resulting solution was then partitioned in a separatory funnel and the toluene solution containing the product was concentrated to contain about 30 mL of toluene plus the product.
Heptane (160 mL) was added and the solution was concentrated to about 60 mL (total volume including the product).
Another 25 mL portion of heptane was added and the organic layer contained NMT 1% toluene by weight.
The solution temperature was kept at or above 30° C. over the solvent swapping operation.
The solution was then warmed briefly to 50° C. or greater to ensure complete dissolution of any crystalline material and was then cooled to 0° C. over 2 h.
The solution was allowed to stir another 2 h at 0° C. and it was filtered at that temperature.
The cake was washed with cold (0° C.) heptane (30 mL) and was allowed to dry in a vacuum drying oven at room temperature for 24 h to afford 32 g of compound 3 (71% unadjusted) as a yellow-orange solid. m.p. 67° C.-69° C. (heptane by DSC), lit=69° C. (Korte, Friedhelm, Annalen der Chemie, Justus Liebigs 1960, V630, pp. 71-83). FT IR (cm-1): 3339 br, 2953, 2928, 2860, 1639, 1581, 1366, 1314, 1256, 1213, 1174, 1105, 1015, 837, 712, 673, 620 (partial reporting).
1H NMR (400 MHz, CDCl3): 11.79 (s,1H); 6.27(d,1H, J=2 Hz); 6.22(d,1H, J=2 Hz); 5.5-5.1 (brs,1H); 4.40(q,2H, J=8 Hz); 2.87-2.83(m,2H); 1.75-1.45(m, 4H); 1.41(t,3H, J=8 Hz); 1.38-1.28(m, 2H); 0.89(t,3H, J=8 Hz).
13C NMR (100.6 MHz, DMSO-d6): 171.9, 165.4, 160.6, 149.2, 111.1, 105.4, 101.7, 61.6, 37.2, 32.3, 31.9, 22.9, 14.4, 14.3.
References:
US2017/349518,2017,A1 Location in patent:Paragraph 0081
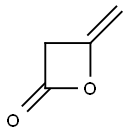
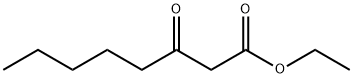

![6-Hydroxy-4-[(2,4-dihydroxy-6-pentylbenzoyl)oxy]-2-pentylbenzoic acid](/CAS/GIF/641-68-9.gif)