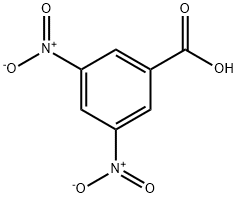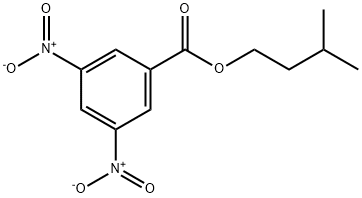
Benzoic acid, 3,5-dinitro-, 3-Methylbutyl ester synthesis
- Product Name:Benzoic acid, 3,5-dinitro-, 3-Methylbutyl ester
- CAS Number:10574-12-6
- Molecular formula:C12H14N2O6
- Molecular Weight:282.25
Yield:10574-12-6 93%
Reaction Conditions:
with potassium carbonate;sodium chloride at 70; under 3150.32 Torr; for 0.0333333 h;Microwave irradiation;Sealed tube;Green chemistry;
Steps:
General procedure for 3,5-dinitrobenzoylation of alcohols/ phenols
General procedure: 3,5-dinitrobenzoyl chloride (10.0 mmol) was blended well with anhydrous K2CO3 (0.75 g, 5.0 mmol) and NaCl (0.75 g) in a mortar pestle. Then reaction mixture was taken in the microwave vessel and alcohol/ phenol (10.0 mmol) was added to it and mixed thoroughly. The reaction vessel was positioned into the microwave reactor under sealed condition with a stirrer and irradiated at a power of 100 W, 110°C and 5 bar (maximum pressure) for 2 mins. During reaction, the reactor read the maximum pressure as 3.2 bar. After completion, the reactor was allowed to run for 10 mins to purge the compressed air to cool the reaction vessel. The reaction mixture was then transferred into ice-water. The solid product was then collected through filtration and washed with cold water. Then 3,5-dinitrobenzoate was purified by crystallization from methanol-water to furnish the product.
References:
Chakraborty, Suchandra;Saha, Ahana;Basu, Kaushik;Saha, Chandan [Synthetic Communications,2015,vol. 45,# 20,p. 2331 - 2343] Location in patent:supporting information