
Benzothiazole, 2-ethyl-6-nitro- (8CI,9CI) synthesis
- Product Name:Benzothiazole, 2-ethyl-6-nitro- (8CI,9CI)
- CAS Number:17142-80-2
- Molecular formula:C9H8N2O2S
- Molecular Weight:208.24
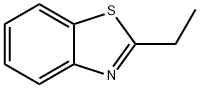
936-77-6
23 suppliers
$151.00/1g

17142-80-2
5 suppliers
inquiry
Yield:17142-80-2 68%
Reaction Conditions:
with sulfuric acid;HNO3 at 0; for 2 h;
Steps:
2-Ethyl-6-nitro-1,3-benzothiazole
To a cooled (0 °C) solution of 2-ethyl-1,3-benzothiazole (2.30 g, 14.1 mmol) in concentrated H2SO4 (10 mL) fuming HNO3 (4.44 g, 70.4 mmol) was added dropwise, and the mixture was stirred 2 h at 0 °C. The mixture was concentrated and poured into ice-water. DCM (100 mL) was added, and the phases were separated. The organic phase was washed with aq NaHCO3 and brine, dried over Na2SO4 and evaporated. The residue was purified by column chromatography (PE/EtOAc = 1/0~0/1, silica-CS 40 g, 40 mL/min, silica gel, UV 254 nm) to afford the product (2.00 g, 68 %). ESI-MS m/z calcd for [C9H8N2O2S] [M+H]+: 209.0; found: 209.1.1H NMR (400 MHz, Chloroform- d) δ 8.75 (d, J = 2.4 Hz, 1H), 8.17 (dd, J = 8.8, 2.0 Hz, 1H), 7.90 (d, J = 8.8 Hz, 1H), 3.14 (q, J = 7.6 Hz, 2H), 1.44 (t, J = 7.6 Hz, 3H).
References:
WO2022/144274,2022,A1 Location in patent:Page/Page column 187
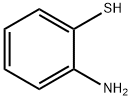
137-07-5
291 suppliers
$10.00/5g

17142-80-2
5 suppliers
inquiry