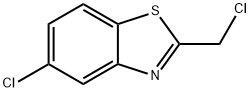
Benzothiazole, 5-chloro-2-(chloromethyl)- (9CI) synthesis
- Product Name:Benzothiazole, 5-chloro-2-(chloromethyl)- (9CI)
- CAS Number:110704-19-3
- Molecular formula:C8H5Cl2NS
- Molecular Weight:218.1
Yield:110704-19-3 51.5%
Reaction Conditions:
with triethylamine in toluene at 20 - 105; for 2.83333 h;
Steps:
5-chloro-2-(chloromethyl)benzo[d]thiazole (33)
To a solution of 2-amino-4-chlorobenzenethiol (8.0 g, 50.1 mmol) in toluene (150 mL), chloroacetyl chloride (11.3 g, 100 mmol) was added drop-wise, followed by the Et3N (7.0 ml, 50 mmol). The mixture was stirred for 20 min at room temperature and then heated to 105oC for another 2.5 h. The reaction was quenched with saturated sodium bicarbonate solution (400 mL). The aqueous phase was extracted with ethyl acetate twice. The combined organic phases were washed with brine, dried over Na2SO4, and concentrated. The resultant residue was purified by flash chromatography. Elution with 10/1 petroleum ether/ethyl acetate gave 5.58 g (51.5%) as a white solid. mp: 102-104;1H-NMR (CDCl3): δ 4.93 (s, 2H), 7.41 (dd, J=8.8, 2.0 Hz, 1H), 7.81 (d, J=8.8 Hz, 1H), 8.01 (d, J=2.0 Hz, 1H).
References:
Hu, Zhongping;Zhang, Shasha;Zhou, Weicheng;Ma, Xiang;Xiang, Guangya [Bioorganic and Medicinal Chemistry Letters,2017,vol. 27,# 8,p. 1854 - 1858] Location in patent:supporting information
74974-54-2
173 suppliers
$6.00/1g
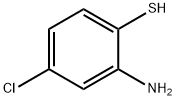
1004-00-8
150 suppliers
$15.00/5g

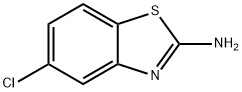
110704-19-3
26 suppliers
inquiry
20358-00-3
151 suppliers
$6.00/250mg

74974-54-2
173 suppliers
$6.00/1g

110704-19-3
26 suppliers
inquiry
51076-95-0
129 suppliers
$43.50/1g

1004-00-8
150 suppliers
$15.00/5g

110704-19-3
26 suppliers
inquiry
