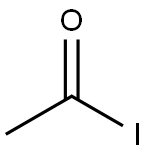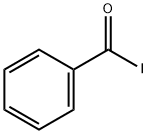
benzoyl iodide synthesis
- Product Name:benzoyl iodide
- CAS Number:618-38-2
- Molecular formula:C7H5IO
- Molecular Weight:232.02
65-85-0
1352 suppliers
$10.00/25g

618-38-2
4 suppliers
inquiry
Yield:618-38-2 100 %Spectr.
Reaction Conditions:
with 1-iodo-N,N,2-trimethylprop-1-en-1-amine in chloroform-d1 at 20;Inert atmosphere;
Steps:
4.2. General procedures for the reaction of a-haloenamines with oxyacids and alcohols
General procedure: Reactions were performed under dry argon atmosphere under magnetic stirring. In procedure A, the alcohol was introduced through a syringe into a 0.5-1 M solution of the α-haloenamine (usually 1.1 equiv) in freshly dried chloroform or dichloromethane or the corresponding deuterated solvents. The reactions were quite exothermic. When performed on a preparative scale, the alcoholwas added at 0 °C, and then the mixture was left at room temperaturefor 0.5-3 h. In procedure B, the α-haloenamine was introduced into a solution of alcohol in the same solvents at 0 °C. It was shown that both procedures gave identical results. In few cases involving the preparation of unstable halides, the halogenation was effected at lower temperature (see Scheme 3) for up to 4 h. Yields were determined after removal of the solvent either by 1H NMR using an added standard (usually benzene or toluene) or by GLC. In some cases the halides were purified by distillation or flash chromatography. The isolated yields were always very close to those measured by NMR or GLC. Most of the halogenation products obtained in this study were known compounds: their spectroscopic properties have been shown to be identical to those reported in the literature and will therefore not been reported here.
References:
Munyemana, Fran?ois;George, Isabelle;Devos, Alain;Colens, Alain;Badarau, Eduard;Frisque-Hesbain, Anne-Marie;Loudet, Aurore;Differding, Edmond;Damien, Jean-Marie;Rémion, Jeanine;Van Uytbergen, Jacqueline;Ghosez, Léon [Tetrahedron,2016,vol. 72,# 3,p. 420 - 430]

98-88-4
578 suppliers
$10.00/5g

618-38-2
4 suppliers
inquiry
111772-04-4
0 suppliers
inquiry

618-38-2
4 suppliers
inquiry

18421-48-2
0 suppliers
inquiry

618-38-2
4 suppliers
inquiry