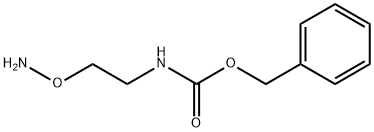
Benzyl (2-(aMinooxy)ethyl)carbaMate synthesis
- Product Name:Benzyl (2-(aMinooxy)ethyl)carbaMate
- CAS Number:226569-28-4
- Molecular formula:C10H14N2O3
- Molecular Weight:210.23
168827-96-1
11 suppliers
inquiry

226569-28-4
11 suppliers
inquiry
Yield:226569-28-4 97%
Reaction Conditions:
with methylamine in methanol;dichloromethane; for 2 h;
Steps:
11 Reference Example 11 Benzyl 2-(aminooxy)ethylcarbamate
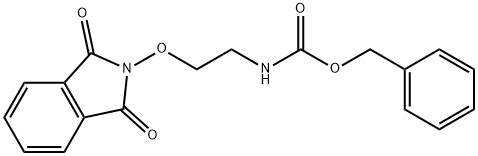
Reference Example 11 Benzyl 2-(aminooxy)ethylcarbamate [0253] To a solution of benzyl (2-((1,3-dioxoisoindolin-2-yl)oxy)ethyl)carbamate (9.36 g, 27.50 mmol) described in Reference Example 10 in methylene chloride (51 mL) was gradually added 9.8M methylamine methanol solution (8.50 mL), followed by stirring for 2 hours. The reaction solution was distilled off under reduced pressure, and methylene chloride (50 mL) and water (80 mL) were added, followed by adjusting to pH 1 with 5M hydrochloric acid, aqueous layer separation, and further washing with methylene chloride (50 mL). To the resulting aqueous layer was added methylene chloride (50 mL), followed by adjusting to pH11 with 5M sodium hydroxide, organic layer separation, and further extracting the aqueous layer with methylene chloride (50 mL) twice. The combined organic layers were washed with 50% potassium carbonate aqueous solution, followed by drying over anhydrous potassium carbonate, and distilling off the solvent under reduced pressure. The resulting residue was subjected to silica gel column chromatography (ethyl acetate) to afford 5.61 g of the title compound (yield 97%). 1H NMR (400 MHz, CDCl3) δ 3.42-3.46 (m, 2H), 3.72-3.74 (m, 2H), 5.10 (s, 2H), 5.15 (br s, 1H), 7.29-7.39 (m, 5H); MS m/z 211 [M+H]+.
References:
EP2857401,2015,A1 Location in patent:Paragraph 0253