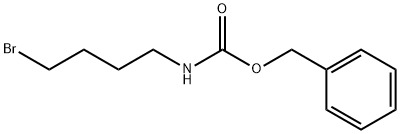
BENZYL (4-BROMOBUTYL)CARBAMATE synthesis
- Product Name:BENZYL (4-BROMOBUTYL)CARBAMATE
- CAS Number:101625-10-9
- Molecular formula:C12H16BrNO2
- Molecular Weight:286.16
558-13-4
253 suppliers
$10.00/1g
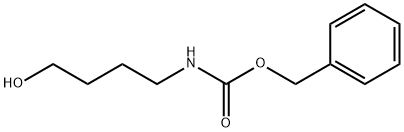
17996-13-3
65 suppliers
$23.00/250mg

101625-10-9
8 suppliers
inquiry
Yield:101625-10-9 99.5%
Reaction Conditions:
with triphenylphosphine in dichloromethane;
Steps:
22.b 6-Amino-2-((2-methyl-1-(3-phenylpropanoylamino)propyl)hydroxyphosphinoyl)methylhexanoic acid
(b) The 4-benzyloxycarbonylamino-1-butanol (8.15 g) obtained in the above step (a) and carbon tetrabromide (15.12 g) were dissolved in methylene chloride (130.4 ml) and cooled with water. The mixture was added with triphenylphosphine (14.4 g) and returned to room temperature, and then stirred for 4 hours. The reaction system was concentrated under reduced pressure without any treatment, and the residue was purified by silica gel column chromatography (400 g, hexane:ethyl acetate=5:2) to obtain 1-benzyloxycarbonylamino-4-bromobutane (10.4 g, 99.5%). 1H-NMR(CDCl3): δ1.58-1.72(2H,m), 1.84-1.94(2H,m), 3.18-3.28(2H,dd), 3.42(2H,dd), 4.80(1H,s), 5.10(2H,s), 7.29-7.39(5H,m). EIMS(m/z): 286(M+).
References:
US6576627,2003,B1

17996-13-3
65 suppliers
$23.00/250mg

101625-10-9
8 suppliers
inquiry
24566-81-2
93 suppliers
$9.00/250mg

501-53-1
393 suppliers
$10.00/1g

101625-10-9
8 suppliers
inquiry
128-08-5
829 suppliers
$5.00/5G

17996-13-3
65 suppliers
$23.00/250mg

101625-10-9
8 suppliers
inquiry

501-53-1
393 suppliers
$10.00/1g

101625-10-9
8 suppliers
inquiry