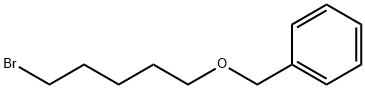
BENZYL 5-BROMOPENTYL ETHER synthesis
- Product Name:BENZYL 5-BROMOPENTYL ETHER
- CAS Number:1014-93-3
- Molecular formula:C12H17BrO
- Molecular Weight:257.17
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

1014-93-3
70 suppliers
$51.00/5g
Yield: 99%
Reaction Conditions:
with sodium bromide in N,N-dimethyl-formamide at 45; for 5 h;Inert atmosphere;
Steps:
1 4.1 [{(5-Bromopentyl)oxy}methyl]benzene (2) [47]
A solution of pentane-1,5-diol (21 mL, 200 mmol) in THF (400 mL) was added drop wise to a stirred suspension of oil free sodium hydride (4.8 g, ∼55% in oil, 200 mmol) in THF (100 mL) under argon atmosphere. The reaction mixture was heated under reflux for 4 h, cooled to room temperature and benzyl bromide (23.7 mL, 200 mmol) was drop wise added to it. The reaction mixture was heated under reflux overnight, cooled to room temperature and concentrated under reduced pressure. The residue was diluted with water and extracted with ethyl acetate. The organic extract was concentrated under reduced pressure and purified by fractional distillation to give 5-(benzyloxy)pentane-1-ol (25.2 g, 65%); bp 140-150 °C (bath) (0.4 mbar) as a colorless liquid. p-Toluenesulphonyl chloride (24.8 g, 130 mmol) was added to a stirred solution of 5-(benzyloxy)pentane-1-ol (25.2 g, 130 mmol) in dry pyridine (30 mL) at 0 °C. The reaction mixture was left standing at 4 °C overnight. The reaction mixture was allowed to attain to room temperature, diluted with water and extracted with 15% ethyl acetate in hexanes. The organic extract was concentrated under reduced pressure to give 5-(benzyloxy)pentyl-1-(4-methyl)benzenesulfonate (42.5 g, 94%) as a colorless gum. Sodium bromide (13 g, 126 mmol) was added to the stirred solution of 5-(benzyloxy)pentyl-1-(4-methyl)benzenesulfonate (42.5 g, 122 mmol) in DMF (300 mL) and the reaction mixture was heated at 45 °C for 5 h. The reaction mixture was diluted with water, and extracted with 10% ethyl acetate in hexanes. The organic extract was concentrated to give bromide 2 (31.3 g, 99%) as a colorless liquid. IR (film): υ 3063, 3029, 2937, 2859, 1646, 1495, 1454, 1362, 1245, 1203, 1103, 1027, 735, 697 cm-1. 1H NMR (200 MHz, CDCl3): δ 1.56-1.73 (m, 4H, 2 × CH2), 1.80-1.95 (m, 2H, CH2CH2Br), 3.41 (t, 2H, 3JHH = 6.8 Hz, OCH2), 3.49 (t, 2H, 3JHH = 6.4 Hz, CH2Br), 4.51 (s, 2H, OCH2Ph), 7.31-7.36 (m, 5H, Ph). 13C NMR (50 MHz, CDCl3): δ 24.9, 28.8, 32.5, 33.7, 69.9, 72.9, 127.5, 127.6 (2C), 128.3 (2C), 138.4.
References:
Paramanik, Minakshmi;Singh, Rekha;Mukhopadhyay, Sulekha;Ghosh, Sunil K. [Journal of Fluorine Chemistry,2015,vol. 178,art. no. 8596,p. 47 - 55]
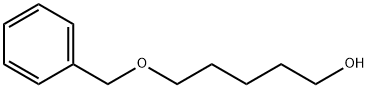
4541-15-5
67 suppliers
$30.00/1g

1014-93-3
70 suppliers
$51.00/5g

821-25-0
2 suppliers
inquiry
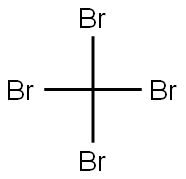
558-13-4
251 suppliers
$10.00/1g

4541-15-5
67 suppliers
$30.00/1g

1014-93-3
70 suppliers
$51.00/5g

