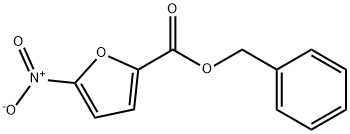
benzyl 5-fluorofuran-2-carboxylate synthesis
- Product Name:benzyl 5-fluorofuran-2-carboxylate
- CAS Number:1333218-13-5
- Molecular formula:C12H9FO3
- Molecular Weight:220.2
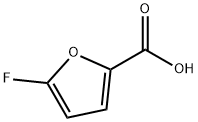
22427-65-2
2 suppliers
inquiry

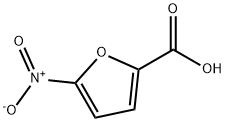
1333218-13-5
6 suppliers
inquiry
Yield:1333218-13-5 59%
Reaction Conditions:
with potassium fluoride;tetraphenylphosphonium bromide in sulfolane at 140; for 2 h;Inert atmosphere;
Steps:
Standard procedure for fluorodenitration: A suspension of commercially available benzyl 5-nitrofuran-2-carboxylate (10c, 0.793 g, 3.21 mmol), spray-dried potassium fluoride (0.932 g, 16.0 mmol), and tetraphenylphosphonium bromide (0.139 g, 0.330 mmol) in anhydrous sulfolane (10 mL) was heated at 140 °C under nitrogen for 2 h (reaction was monitored by TLC). The reaction mixture was cooled to rt and diluted with water (10 mL). The resulting mixture was extracted with diethyl ether (×3). The combined extracts were washed with brine, dried over anhydrous MgSO4, filtered, and concentrated under reduced pressure. The resulting residue was purified by flash column (5% MTBE/hexanes) to give 11c (0.416 g, 59%) as a colorless oil: 1H NMR (300 MHz, CDCl3) δ 7.40-7.34 (m, 5H), 7.15 (t, J = 3.5 Hz, 1H), 5.62 (dd, J = 7.1, 3.6 Hz, 1H), 5.32 (s, 2H); 13C NMR (75 MHz, CDCl3) δ 158.9 (d, J = 284 Hz), 157.7, 135.5, 135.0, 128.6, 128.5, 128.4, 120.6, 85.1 (d, J = 13.4 Hz), 66.6; 19F NMR (282 MHz, CDCl3) δ 106.4.
References:
Song, Renhua;Lin, Weimin;Jiang, Qin [Tetrahedron Letters,2011,vol. 52,# 38,p. 4965 - 4966] Location in patent:experimental part