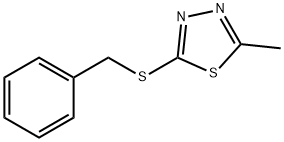
benzyl 5-methyl-1,3,4-thiadiazol-2-yl sulfide synthesis
- Product Name:benzyl 5-methyl-1,3,4-thiadiazol-2-yl sulfide
- CAS Number:42755-32-8
- Molecular formula:C10H10N2S2
- Molecular Weight:222.33

100-39-0
431 suppliers
$10.00/10g
![1,3,4-Thiadiazole, 2-methyl-5-[(trimethylsilyl)thio]-](/CAS/20180703/GIF/81589-00-6.gif)
81589-00-6
2 suppliers
inquiry
29490-19-5
406 suppliers
$6.00/10g
81-07-2
440 suppliers
$13.98/25G

42755-32-8
1 suppliers
inquiry
Yield:42755-32-8 96.4%
Reaction Conditions:
with 1,1,1,3,3,3-hexamethyl-disilazane in N,N,N,N,N,N-hexamethylphosphoric triamide;water;ethyl acetate;acetonitrile;
Steps:
17 EXAMPLE 17
EXAMPLE 17 15 ml (72 mmoles) of hexamethyldisilazane were added to a refluxing mixture of 9.9 g (75 mmoles) of 5-mercapto-2-methyl-1,3,4-thiadiazole, 27 mg (0.15 mmole) of saccharin, 50 ml of acetonitrile and 20 ml of hexamethylphosphoric triamide and after refluxing for 4.5 hours, the calculated amount of ammonia had been evolved. Refluxing was continued for 30 minutes and the mixture containing 5-trimethylsilylthio-2-methyl-1,3,4-thiadiazole was cooled to room temperature and 9.9 ml (80 mmoles) of benzyl bromide were added thereto which raised the temperature to about 50° C. All starting material was consumed within 5 minutes. The acetonitrile was removed by evaporation and 100 ml of ethyl acetate were added to the residue. The solution was poured into 250 ml of water and the aqueous layer was extracted three times with 30 ml of ethyl acetate. The combined organic extracts were washed with a 10% sodium chloride solution, dried, filtered and evaporated to dryness. The solid residue was washed with hexane containing some diethyl ether to obtain 15.91 g (96.4% yield) of 5-benzylthio-2-methyl-1,3,4-thiadiazole melting at 60°-63° C. Crystallization from a mixture of ethyl acetate and petroleum ether (boiling range 40°-60° C.) provided 14.27 g of the pure substance melting at 62.5°-63.5° C.
References:
US4496720,1985,A

100-44-7
639 suppliers
$13.50/250G

29490-19-5
406 suppliers
$6.00/10g

42755-32-8
1 suppliers
inquiry
![Phosphoramidic acid, [(4-methylphenyl)sulfonyl]-, bis(4-nitrophenyl) ester (9CI)](/CAS/20210305/GIF/81589-21-1.gif)
81589-21-1
0 suppliers
inquiry

100-39-0
431 suppliers
$10.00/10g

29490-19-5
406 suppliers
$6.00/10g

42755-32-8
1 suppliers
inquiry

100-44-7
639 suppliers
$13.50/250G

42755-32-8
1 suppliers
inquiry