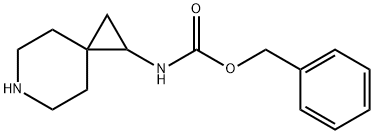
benzyl (6-Azaspiro[2.5]octan-1-yl)carbaMate synthesis
- Product Name:benzyl (6-Azaspiro[2.5]octan-1-yl)carbaMate
- CAS Number:1239852-33-5
- Molecular formula:C15H20N2O2
- Molecular Weight:260.33
![tert-Butyl 1-benzyloxycarbonylaMino-6-azaspiro[2.5]octane-6-carboxylate](/CAS/20150408/GIF/1239852-32-4.gif)
1239852-32-4
17 suppliers
$740.00/1g
![benzyl (6-Azaspiro[2.5]octan-1-yl)carbaMate](/CAS/GIF/1239852-33-5.gif)
1239852-33-5
23 suppliers
inquiry
Yield:1239852-33-5 48%
Reaction Conditions:
with trifluoroacetic acid in dichloromethane at 20; for 1.75 h;
Steps:
15.b Step b:
TFA (10 mL, 131 mmol) was added to a solution tert-butyl 1- (benzyloxycarbonylamino)-6-azaspiro[2.5]octane-6-carboxylate (945 mg, 2.62 mmol) in DCM (13 mL) at room temperature. The mixture was stirred for 1h45 and the volatiles were removed under reduced pressure. The resulting oil was dissolved in DCM (25 mL). A saturated aqueous solution of NaHCO3 (25 mL) was slowly added followed by water (25 mL). The two layers were separated and the aqueous layer was extracted with more DCM (4 x 50 mL). The combined organic layers were washed with brine (25 mL), dried over anhydrous MgSO4, filtrated and the volatiles were removed under reduced pressure to give benzyl 6- azaspiro[2.5]octan-1-ylcarbamate (327 mg, 48% yield) as a beige foam.1H NMR (500 MHz, DMSO-d6) δ 7.44- 7.25 (m, 5H), 5.14- 4.92 (m, 2H), 2.88- 2.59 (m, 4H), 2.35 (dt, J = 8.0, 4.0 Hz, 1H), 1.47- 1.14 (m, 4H), 0.63- 0.52 (m, 1H), 0.43- 0.28 (m, 1H).2 NH missing. MS (ES+) m/z 261 (M+1).
References:
WO2018/57884,2018,A1 Location in patent:Paragraph 0176