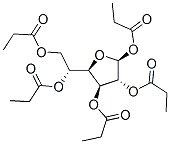
.beta.-D-Glucofuranose, pentapropanoate synthesis
- Product Name:.beta.-D-Glucofuranose, pentapropanoate
- CAS Number:294638-87-2
- Molecular formula:C21H32O11
- Molecular Weight:460.47
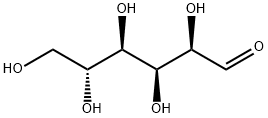
50-99-7
989 suppliers
$6.00/25g

802294-64-0
1 suppliers
inquiry
123-62-6
305 suppliers
$17.00/25g

294638-87-2
3 suppliers
inquiry
Yield:-
Reaction Conditions:
with boric acid
Steps:
1 1,2,3,5,6-Penta-O-propanoyl-β-D-glucofuranose
Example 1 1,2,3,5,6-Penta-O-propanoyl-β-D-glucofuranose D-Glucose (5.0 g, 27.8 mmol), boric acid (3.5 g, 56.9 mmol, 2.05 eq.) and propanoic acid (75 mL) were stirred at 70° C. for one hour. By this time, all the D-glucose had dissolved. Propanoic anhydride (75 mL) was added slowly and the resulting mixture was heated at 70° C. for 48 hours. The reaction mixture was diluted to 1000 mL with ice (100 g) and water and the mixture stirred vigorously for one hour. The resulting precipitate was collected by filtration and recrystallized twice from ethanol (20 mL) to give the title compound as white prisms (7.4 g, 58%); mp 74.5-75.5° C., [α]D22-30.8 (c 5.0, CHCl3). IR (CDCl3) 2987, 1740 cm-1. 1H NMR (CDCl3): δ 6.14 (s, 1H, H1), 5.42 (d, 1H, J=4.8 Hz, H3), 5.29 (ddd, 1H, J=9.4, 5.2, 2.5, Hz, H5), 5.10 (s, 1H, H2), 4.63 (dd, 1H, J=12.3, 2.5 Hz, H6a), 4.55 (dd, 1H, J=9.4, 4.7 Hz, H4), 4.10 (dd, 1H, J=12.3, 5.2 Hz, H6b), 2.40 (q, 2H, J=7.6 Hz), 2.38 (q, 2H, J=7.6 Hz), 2.37 (q, 2H, J=7.6 Hz), 2.34 (q, 2H, J=7.6 Hz), 2.26 (q, 2H, J=7.6 Hz) (5*CH2), 1.17 (t, 6H, J=7.6 Hz), 1.13 (t, 6H, J=7.6 Hz), 1.09 (t, 3H, J=7.6 Hz) (5*CH3) ppm. 13C NMR (CDCl3): δ 174.3, 173.4, 173.0, 172.9 (2) (5*C=O), 99.2 (C1), 80.2 (C4), 79.9 (C2), 73.2 (C3), 68.6 (C5), 63.3 (C6), 28.0, 27.7 (3), 27.6 (5*CH2), 9.4, 9.2 (2), 9.1 (2) (5*CH3) ppm. LRMS (FAB) 387 (M+-C3H5O2, 100%), 183 (24), 137 (25), 57 (52) m/z. HRMS (FAB): calcd for C21H32O11 (M+-H) 459.186360, found 459.186637. Anal: calcd for C21H32O11: C 54.78; H 7.00. Found: C 54.80; H 6.96. The perpropanoylated glucofuranose of Example 1 was obtained in crystalline form and in greater than approximately 90% anomeric purity. The compound is therefore desirable is a starting material for the synthesis of other compounds containing chiral carbon atoms.
References:
US6620921,2003,B1
