beta-D-Glucose synthesis
- Product Name:beta-D-Glucose
- CAS Number:106245-04-9
- Molecular formula:C13H11FO2
- Molecular Weight:218.22
202857-89-4
52 suppliers
$16.00/100mg

106245-04-9
5 suppliers
inquiry
Yield:106245-04-9 78%
Reaction Conditions:
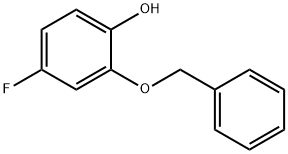
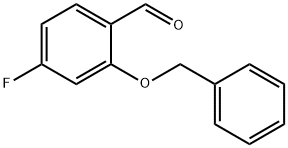
Stage #1: 2-(benzyloxy)-4-fluorobenzaldehydewith 3-chloro-benzenecarboperoxoic acid in dichloromethane at 40;
Stage #2: with lithium hydroxide in tetrahydrofuran at 20; for 1 h;
Steps:
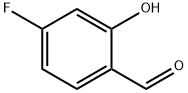
B B. 2-(Benzyloxy)-4-fluorophenol
3-Chlorobenzoperoxoic acid (14.0 g, 81 .0 mmol) was added to a solution of 2- (benzyloxy)-4-fluorobenzaldehyde (Intermediate 2A) (7.50 g, 32.6 mmol) in DCM (100 mL) and stirred at 40 °C overnight, cooled and washed with saturated NaHC03 (3X) and brine. The organic layer was dried over Na2S04 and evaporated. The residue was dissolved in THF (100 mL) and water (50 mL), and lithium hydroxide (2.34 g, 98.0 mmol) was added. The reaction mixture was stirred at room temperature for 1 h. Citric acid was added until pH neutral, and the aqueous phase was extracted with ethyl acetate (3X). The combined organic layers were washed with brine, dried over Na2S04 and evaporated. The residue was purified on silica gel eluting with a 0%-40% EtOAc in hexanes gradient. The appropriate fractions were combined, evaporated under reduced pressure and placed in vacuo to give the title compound (5.5 g, 78%). 1H NMR (400 MHz, CD3SOCD3) δ 5.1 1 (s, 2 H), 6.53-6.59 (m, 1 H), 6.74-6.78 (m, 1 H), 6.87-6.91 (m, 1 H), 7.31 -7.36 (m, 1 H), 7.39-7.42 (m, 2 H), 7.45-7.49 (m, 2 H), 8.95 (s, 1 H); LC-MS (LC-ES) M-H = 217.
References:
WO2018/69863,2018,A1 Location in patent:Page/Page column 101

100-39-0
421 suppliers
$10.00/10g

106245-04-9
5 suppliers
inquiry
348-28-7
192 suppliers
$21.21/250mgs:

106245-04-9
5 suppliers
inquiry