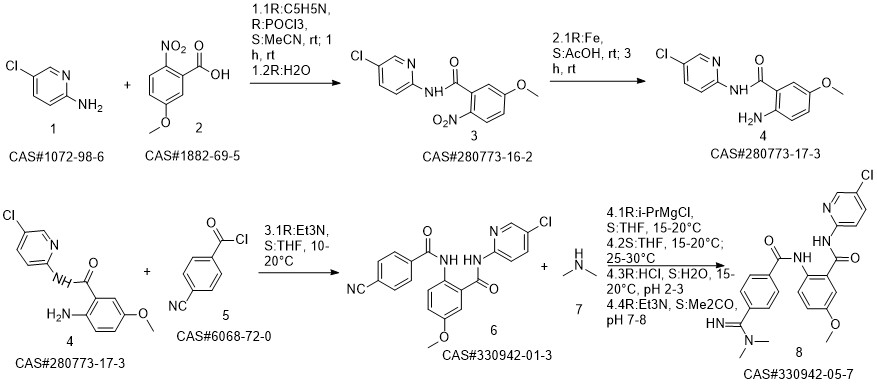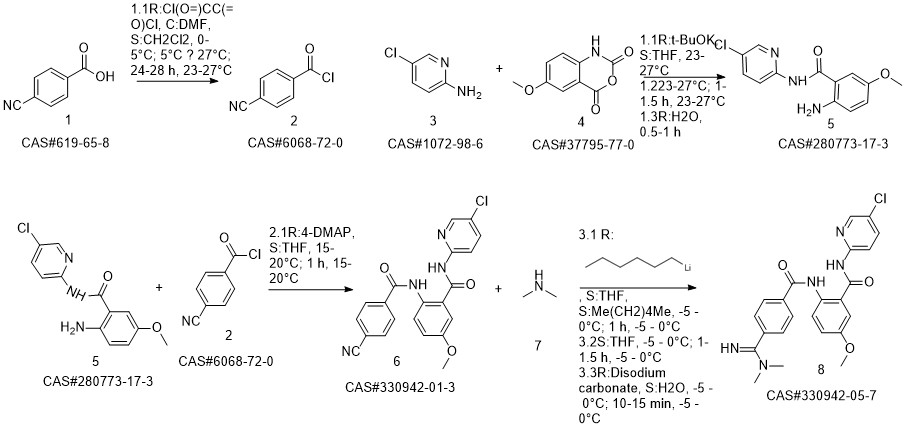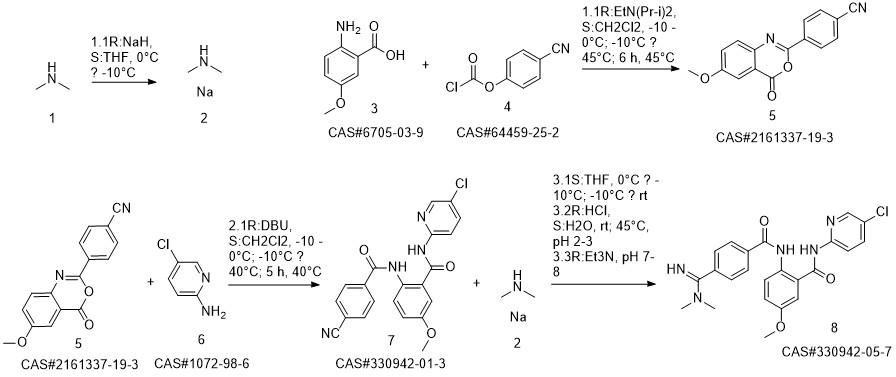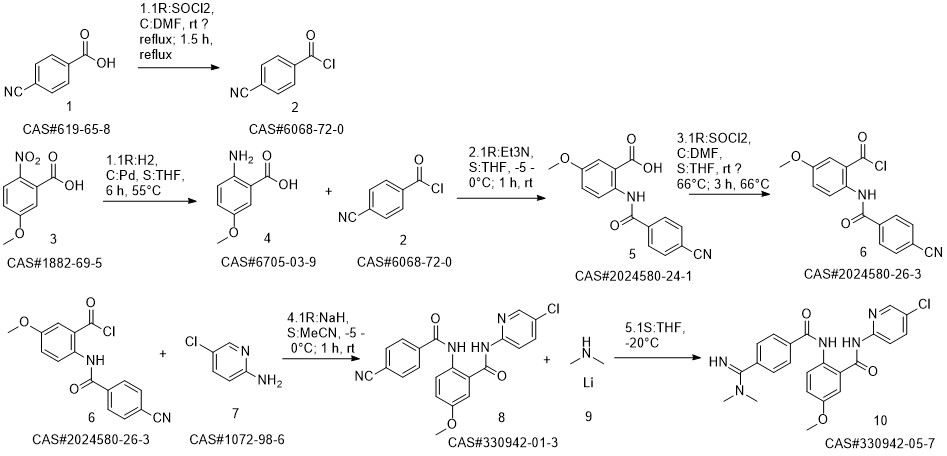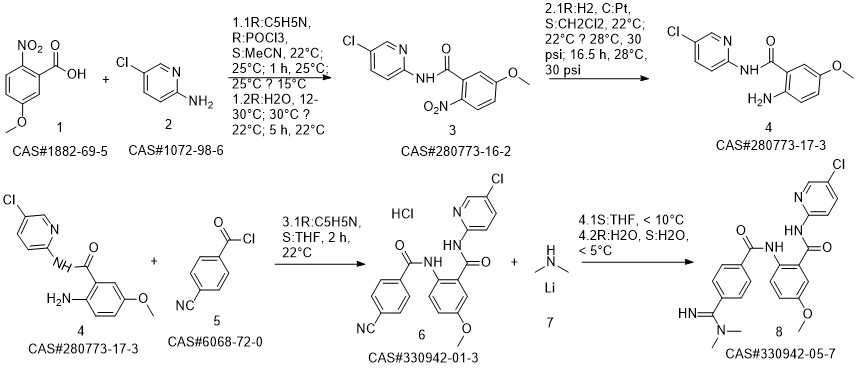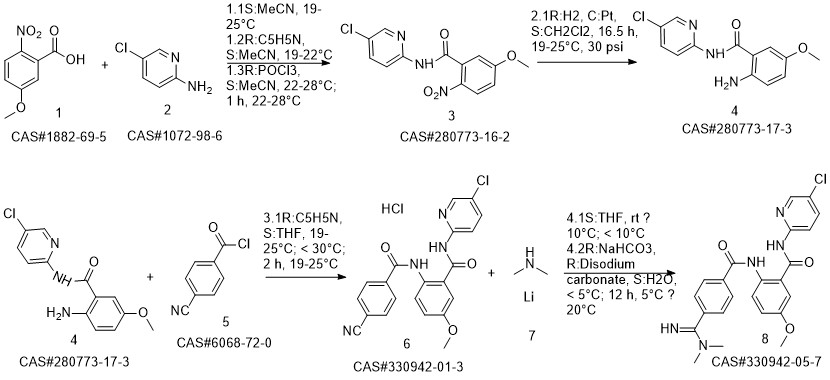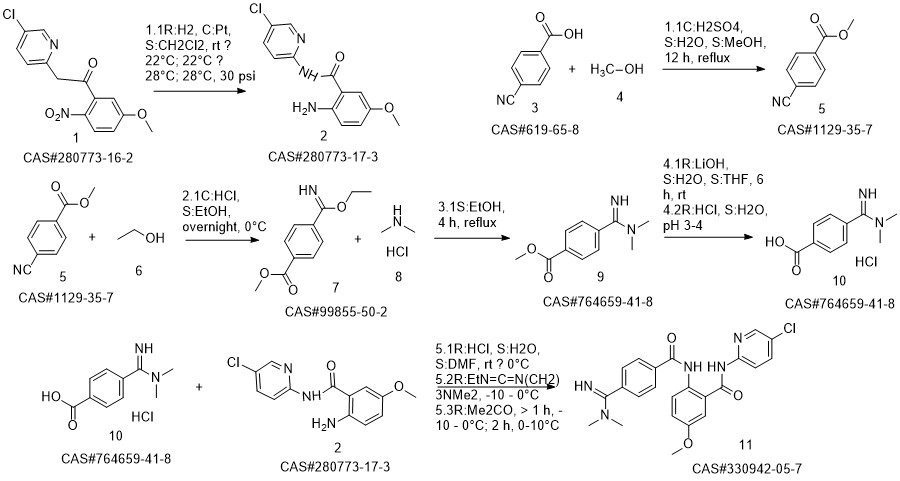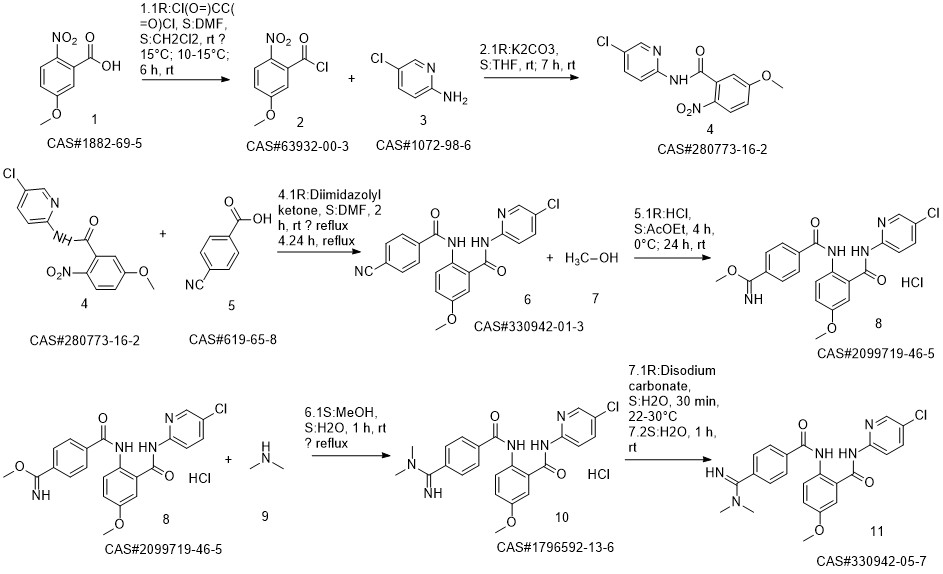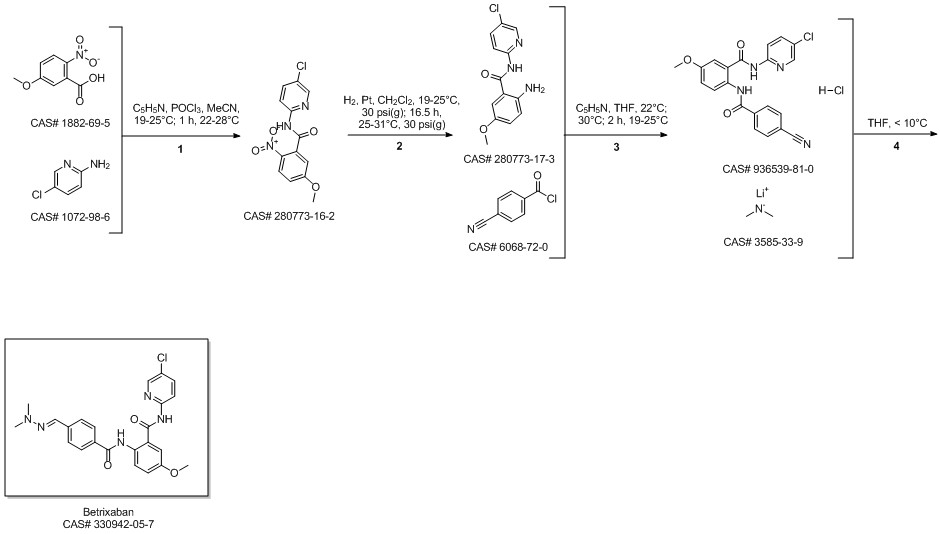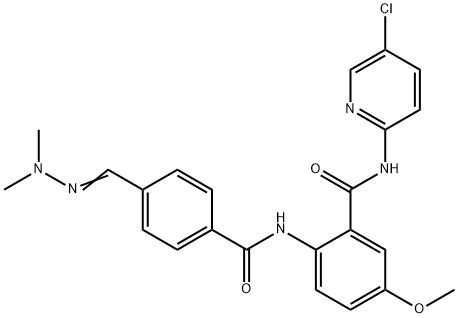
Betrixaban synthesis
- Product Name:Betrixaban
- CAS Number:330942-05-7
- Molecular formula:C23H22ClN5O3
- Molecular Weight:451.91
Reference: Zhang P, Huang W, Wang L, Bao L, Jia ZJ, Bauer SM, Goldman EA, Probst GD, Song Y, Su T, Fan J, Wu Y, Li W, Woolfrey J, Sinha U, Wong PW, Edwards ST, Arfsten AE, Clizbe LA, Kanter J, Pandey A, Park G, Hutchaleelaha A, Lambing JL, Hollenbach SJ, Scarborough RM, Zhu BY. Discovery of betrixaban (PRT054021), N-(5-chloropyridin-2-yl)-2-(4-(N,N-dimethylcarbamimidoyl)benzamido)-5-methoxybenz amide, a highly potent, selective, and orally efficacious factor Xa inhibitor. Bioorg Med Chem Lett. 2009 Apr 15;19(8):2179-85. doi: 10.1016/j.bmcl.2009.02.111. Epub 2009 Mar 3. PubMed PMID: 19297154.
Yield:330942-05-7 90.64%
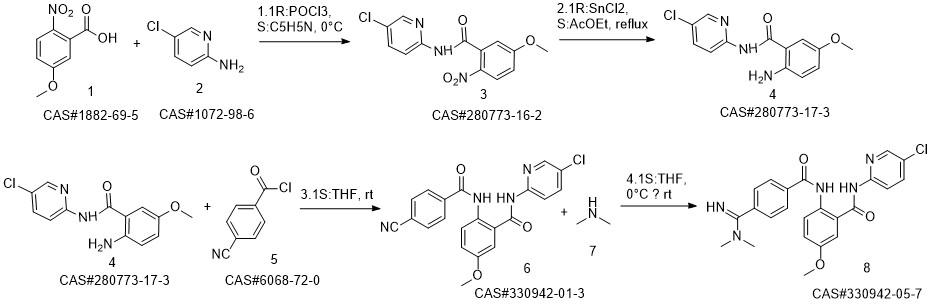
Reaction Conditions:
with dimethyl amine at -5 - 0; for 0.5 h;
Steps:
9 Example 9 Preparation of Betrixaban
Put 350ml of liquefied dimethylamine into the reaction flask,30g compound of formula II (0.074mol),Cool to -5 - 0 °C,Stir,The dimethylaminomagnesium chloride solution (0.5mol) prepared in Example 3 was added dropwise,After dripping for 30 minutes,Raise the temperature to 10-15 to recover dimethylamine,After recycling,Add 300ml of sodium carbonate (30g) and sodium bicarbonate (30g) aqueous solution,Stir for 1h,filter.The solid is slurried twice with methanol,After drying, betrixaban (30.2 g, yield 90.64%, purity 99.6%) was obtained.
References:
CN113620869,2021,A Location in patent:Paragraph 0022; 0079-0083
![N-(5-Chloro-2-pyridinyl)-2-[(4-cyanobenzoyl)amino]-5-methoxybenzamide](/CAS/20150408/GIF/330942-01-3.gif)
330942-01-3
49 suppliers
inquiry

124-40-3
545 suppliers
$18.00/100ml

330942-05-7
186 suppliers
$7.00/1mg
![N-(5-Chloro-2-pyridinyl)-2-[(4-cyanobenzoyl)amino]-5-methoxybenzamide](/CAS/20150408/GIF/330942-01-3.gif)
330942-01-3
49 suppliers
inquiry

330942-05-7
186 suppliers
$7.00/1mg
![N-(5-Chloro-2-pyridinyl)-2-[(4-cyanobenzoyl)amino]-5-methoxybenzamide hydrochloride](/CAS/20150408/GIF/936539-81-0.gif)
936539-81-0
8 suppliers
inquiry

124-40-3
545 suppliers
$18.00/100ml

330942-05-7
186 suppliers
$7.00/1mg

1882-69-5
289 suppliers
$6.00/5g

330942-05-7
186 suppliers
$7.00/1mg