
(+)-Bicifadine Hydrochloride synthesis
- Product Name:(+)-Bicifadine Hydrochloride
- CAS Number:66504-82-3
- Molecular formula:C12H16ClN
- Molecular Weight:209.7151
![3-Azabicyclo[3.1.0]hexane, 1-(4-methylphenyl)-, (1R,5S)-](/CAS/20210305/GIF/83213-66-5.gif)
83213-66-5
1 suppliers
inquiry

66504-82-3
9 suppliers
inquiry
Yield:66504-82-3 87%
Reaction Conditions:
with hydrogenchloride in ethyl acetate;isopropyl alcohol at 20;Product distribution / selectivity;
Steps:
III.C.2
Method 2; EPO
References:
WO2006/96810,2006,A2 Location in patent:Page/Page column 60-61
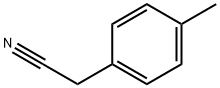
2947-61-7
254 suppliers
$8.00/5g

66504-82-3
9 suppliers
inquiry