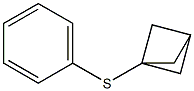
Bicyclo[1.1.1]pentan-1-yl(phenyl)sulphane synthesis
- Product Name:Bicyclo[1.1.1]pentan-1-yl(phenyl)sulphane
- CAS Number:98585-81-0
- Molecular formula:C11H12S
- Molecular Weight:176.278
Yield:98585-81-0 100%
Reaction Conditions:
in diethyl ether at 20; for 0.25 h;
Steps:
[1.1.1]Propellane (1) quantification:
A 1 M thiophenol solution in diethyl ether (165 mg,1.50 mL, 1.5 mmol) was added to 1 mL of the propellane (1) solution and the mixturewas stirred for 15 min at rt. The solution was diluted with 10 mL of pentane andwashed with 10 mL of 1 M NaOH solution. The organic layer was dried by theaddition of Na2SO4. The mixture was filtered through a glass funnel and the solventwas evaporated under reduced pressure to give the target compound 5a (0.0920 g,0.522 mmol) as a colorless oil.The yield of the reaction is assumed to be quantitative according to K. B. Wiberg, S.T. Waddell, J. Am. Chem. Soc. 1990, 112, 2194-2216.
References:
B?r, Robin M.;Heinrich, Gregor;Nieger, Martin;Fuhr, Olaf;Br?se, Stefan [Beilstein Journal of Organic Chemistry,2019,vol. 15,p. 1172 - 1180] Location in patent:supporting information
98577-44-7
216 suppliers
$10.00/1g
![Bicyclo[1.1.1]pentan-1-yl(phenyl)sulphane](/CAS/20180703/GIF/98585-81-0.gif)
98585-81-0
12 suppliers
$503.19/5MG
![Tricyclo[1.1.1.01,3]pentane](/CAS/GIF/35634-10-7.gif)
![bicyclo[1.1.1]pentane](/CAS/20180702/GIF/311-75-1.gif)