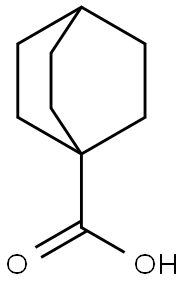
bicyclo[2.2.2]octane-1-carboxylic acid synthesis
- Product Name:bicyclo[2.2.2]octane-1-carboxylic acid
- CAS Number:699-55-8
- Molecular formula:C9H14O2
- Molecular Weight:154.21
![BICYCLO[2.2.2]OCTANE-1,4-DICARBOXYLIC ACID HEMIMETHYL ESTER](/CAS/GIF/18720-35-9.gif)
18720-35-9
232 suppliers
$19.00/1g
![bicyclo[2.2.2]octane-1-carboxylic acid](/CAS/GIF/699-55-8.gif)
699-55-8
65 suppliers
$45.00/10mg
Yield:699-55-8 83%
Reaction Conditions:
Stage #1:bicyclo[2.2.2]octane-1,4-dicarboxylic acid monomethyl ester with tributylphosphine;2,2'-dipyridyl disulfide bis-N-oxide in dichloromethane at 0; for 2 h;
Stage #2: with 2-methylpropan-2-thiol in dichloromethane at 0; for 1.25 h;UV-irradiation;
Steps:
25.1.a 1) Step a: Synthesis of bicyclo[2.2.2]octane-l-carboxylic acid (49)
A flask was charged with 47 (35 g, 165 mmol), 48 (50.0 g, 198 mmol), and DCM (1.5 L). The flask was masked with foil to reduce ambient light. The resulting suspension was cooled to OoC and treated with tributylphosphine (51 mL, 206 mmol) drop wise. The ice bath was removed and stirring continued for 2h. The reaction was cooled to OoC and treated with 2- methylpropane-2-thiol (165 mL, 1.46 mol). The reaction was irradiated with a 300W (0363) Tungstern lamp for 1.25h. The reaction was quenched by addition of a suspension of 350 g calcium hypochlorite in water (2.0L). The mixture was diluted with ether and stirred at 0°C for 5min, followed by room temperature for 20 min. Celite was added to aid in separation of the layers, and the resulting mixture filtered. The eluent was poured into a separatory funnel and the layers separated. The organics were washed with brine, dried over Na2S04, and concentrated. The resulting residue was treated with a solution of 75 g potassium hydroxide in 1 0L methanol/water (1:1). The resulting mixture was stirred at room temperature overnight. The reaction was concentrated to remove most of the methanol and extracted with EtOAc (500 mL x 2) to remove byproducts. The aqueous was made acidic by addition of con.HCl upon which a white precipitate was formed. The precipitate was collected by filtration to afford 49 (21 g, 83%).1H NMR (300 MHz, DMSO-d6), ^ = 1.60 (m, 13H).
References:
STEALTH BIOTHERAPEUTICS CORP.;ZHENG, Guozhu;BAMBERGER, Mark, J.;SMUKSTE, Inese WO2020/131282, 2020, A1 Location in patent:Page/Page column 83; 84
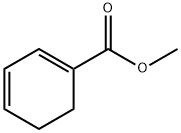
![Bicyclo[2.2.2]oct-2-ene-1-carboxylic acid, methyl ester](/CAS/20210111/GIF/2534-81-8.gif)
2534-81-8
0 suppliers
inquiry
![bicyclo[2.2.2]octane-1-carboxylic acid](/CAS/GIF/699-55-8.gif)
699-55-8
65 suppliers
$45.00/10mg
30810-15-2
0 suppliers
inquiry
![bicyclo[2.2.2]octane-1-carboxylic acid](/CAS/GIF/699-55-8.gif)
699-55-8
65 suppliers
$45.00/10mg
![{bicyclo[2.2.2]octan-1-yl}methanol](/CAS/20200119/GIF/2574-42-7.gif)
2574-42-7
33 suppliers
$155.00/100mg
![Bicycyclo[2,2,2]octane-1-carbaldehyde](/CAS/20210111/GIF/2064-05-3.gif)
2064-05-3
3 suppliers
inquiry
![bicyclo[2.2.2]octane-1-carboxylic acid](/CAS/GIF/699-55-8.gif)
699-55-8
65 suppliers
$45.00/10mg
![methyl bicyclo[2.2.2]octane-1-carboxylate](/CAS/20180629/GIF/2064-04-2.gif)
2064-04-2
13 suppliers
inquiry
![bicyclo[2.2.2]octane-1-carboxylic acid](/CAS/GIF/699-55-8.gif)
699-55-8
65 suppliers
$45.00/10mg