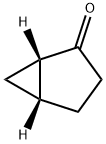
Bicyclo[3.1.0]hexan-2-one, (1S,5R)- synthesis
- Product Name:Bicyclo[3.1.0]hexan-2-one, (1S,5R)-
- CAS Number:196488-92-3
- Molecular formula:C6H8O
- Molecular Weight:96.13
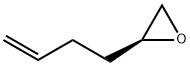
137688-21-2
76 suppliers
$25.00/1g
![Bicyclo[3.1.0]hexan-2-one, (1S,5R)-](/CAS/20180601/GIF/196488-92-3.gif)
196488-92-3
21 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: (-)-(S)-2-(but-3-enyl)oxiranewith 2,2,6,6-tetramethyl-piperidine;hydrogenchloride;n-butyllithium in hexane;tert-butyl methyl ether;water at -12 - 0; for 2 h;Cooling with acetone-dry ice;
Stage #2: with sodium hypochlorite;dipotassium hydrogenphosphate;potassium dihydrogenphosphate;2,2,6,6-Tetramethyl-1-piperidinyloxy free radical;potassium bromide in hexane;tert-butyl methyl ether;water at -20 - 0; for 2.16667 h;Cooling with isopropanol-dry ice;
Steps:
1.17.A
Step A: Preparation of (lS,5/?)-Bicyclo[3.1.0]hexan-2-one.To a stirred solution of (5)-2-(but-3-enyl)oxirane (100 g, 1019 mmol) and 2,2,6,6- tetramethylpiperidine (86 mL, 509 mmol) in MTBE (1000 mL) cooled in a dry ice/acetone bath, was added dropwise a 2.5 M hexane solution of BuLi (489 mL, 1223 mmol) at a rate to maintain the internal temperature at -12 to -5 °C (time of addition = 1 h). After addition was complete, the reaction was stirred one hour at -5 to 0 °C and quenched with 3 M aqueous HQ (545 mL) dropwise (internal temperature rose to 3 °C). The layers were separated and the organic layer was washed with 3 M HC1 (200 mL). The combined aqueous layers were extracted with MTBE (2 x 500 mL). The combined organic layers were washed with brine (3 x 300 mL) and concentrated to give a pale yellow solution (ca. 1000 mL). To this solution in a 5 L 3-neck round bottom flask equipped with an overhead stirrer was added an aqueous solution of dibasic potassium phosphate (216 g, 1240 mmol), monobasic potassium phosphate (12.8 g, 94 mmol), and potassium bromide (18.19 g, 153 mmol) in water (407 mL). The mixture was cooled to -20 °C in a dry-ice/isopropanol bath. TEMPO (4.30 g, 27.5 mmol) was added. The temperature was allowed to warm to 0 °C and aqueous sodium hypochlorite (1.54 M, 1059 mL, 1630 mmol) was added dropwise over 70 min while maintaining the internal temperature between -10 and 0 °C. Stirring was continued at 0 °C for another hour. Sodium sulfite (50 g) was added to quench excess sodium hypochlorite (temperature rose to 12 °C). The layers were separated and the aqueous layer was extracted twice more with MTBE (500 mL then 250 mL). The combined organic layers (total volume ca. 1600 mL) were dried (MgS04) then filtered. The solution was concentrated (ca. 300 mL). The residue was distilled (2 torr/36 °C, note: receveiving flask was cooled in dry ice/acetone bath) to give the title compound (65.8 g) as a light orange oil. .H NMR (400 MHz, CDC13) δ 0.93 (td, / = 4.6, 3.3 Hz, 1H), 1.20 (td, = 8.0, 4.8 Hz, 1H), 1.74-1.79 (m, 1H), 1.98-2.19 (m, 5H).
References:
WO2012/116279,2012,A1 Location in patent:Page/Page column 96
1374009-33-2
14 suppliers
inquiry
![Bicyclo[3.1.0]hexan-2-one, (1S,5R)-](/CAS/20180601/GIF/196488-92-3.gif)
196488-92-3
21 suppliers
inquiry
![(1R,5S)-bicyclo[3.1.0]hexan-2-one](/CAS/20180527/GIF/58001-78-8.gif)