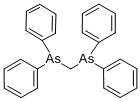
BIS(1,2-DIPHENYLARSENO)METHANE synthesis
- Product Name:BIS(1,2-DIPHENYLARSENO)METHANE
- CAS Number:21892-63-7
- Molecular formula:C25H22As2
- Molecular Weight:472.29

75-09-2
1202 suppliers
$10.00/25ML
20738-31-2
0 suppliers
inquiry
591-51-5
127 suppliers
$123.00/50ml

21892-63-7
11 suppliers
inquiry
Yield:21892-63-7 54%
Reaction Conditions:
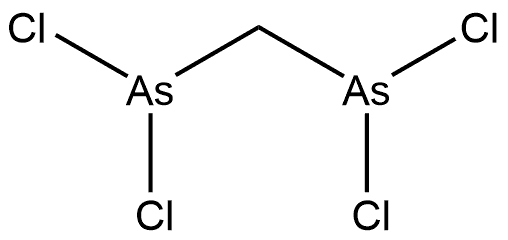
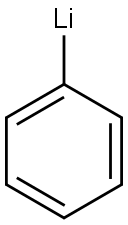
Stage #1: hexaphenylcyclohexaarsane;phenyllithium in tetrahydrofuran at 0 - 20; for 2 h;Inert atmosphere;
Stage #2: dichloromethane in tetrahydrofuran at 0 - 20; for 19 h;Inert atmosphere;
Steps:
Bis(diphenylarsino)methane (dpam)
A THF dispersion (20 mL) of Ph6As6 (787 mg, 0.863 mmol) was cooled to 0 °C under N2 atmosphere, and PhLi (1.6 M in butyl ether, 3.3 mL, 5.2 mmol) was slowly added. The reaction mixture was warmed to room temperature, and stirred for 2 h. Again, the reaction mixture was cooled to 0 °C, and CH2Cl2 (160 mL, 2.6 mmol) was slowly added. After stirring at room temperature for 19 h, the reaction was quenched by distilled water. The resultant solution was extracted with CHCl3 three times, and the combined organic layers were dried over MgSO4. After filtration, the volatiles were removed in vacuo. The residue was subjected to recrystallization from CH2Cl2 and MeOH to obtain colorless crystals of dpam (661 mg, 1.40 mmol, 54%). 1H NMR (CDCl3, 400 MHz) δ 7.43-7.40 (m, 8H), 7.30-7.27 (m, 12H), 2.60 (s, 2H) ppm. This 1H-NMR spectrum corresponds precisely to that of the previous literature.[10] HRMS (FAB) m/z: calcd for C25H22As2 [M]+: 472.0153, found 472.0165.
References:
Imoto, Hiroaki;Konishi, Masafumi;Nishiyama, Shintaro;Sasaki, Hiroshi;Tanaka, Susumu;Yumura, Takashi;Naka, Kensuke [Chemistry Letters,2019,vol. 48,# 11,p. 1312 - 1315] Location in patent:supporting information

75-09-2
1202 suppliers
$10.00/25ML
21498-51-1
0 suppliers
inquiry

603-32-7
105 suppliers
$36.39/5g

21892-63-7
11 suppliers
inquiry
![Arsine oxide, [(diphenylarsino)methyl]diphenyl- (9CI)](/CAS/20210305/GIF/856837-45-1.gif)
856837-45-1
0 suppliers
inquiry

21892-63-7
11 suppliers
inquiry

712-48-1
9 suppliers
inquiry

21892-63-7
11 suppliers
inquiry