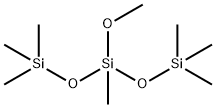
BIS(TRIMETHYLSILOXY)METHYLMETHOXYSILANE synthesis
- Product Name:BIS(TRIMETHYLSILOXY)METHYLMETHOXYSILANE
- CAS Number:7671-19-4
- Molecular formula:C8H24O3Si3
- Molecular Weight:252.53

1185-55-3
460 suppliers
$6.00/25g

107-46-0
460 suppliers
$10.00/10g

17928-28-8
105 suppliers
$108.00/25mL

7671-19-4
32 suppliers
inquiry

5272-21-9
1 suppliers
inquiry
Yield:17928-28-8 91.5%
Reaction Conditions:
Stage #1: Hexamethyldisiloxanewith methanol;sulfuric acid at 5 - 10; for 1 h;
Stage #2: Methyltrimethoxysilan at 5 - 10; for 1.5 - 1.75 h;
Stage #3: with sulfuric acid;waterProduct distribution / selectivity;more than 3 stages;
Steps:
2; 3
Example 2 A 1000-ml four necked glass flask equipped with a reflux condenser, thermometer and stirrer was purged with nitrogen. The flask was charged with 324.8 g (2.0 mol) of hexamethyldisiloxane and 64.0 g (2.0 mol) of methanol, and cooled in an ice water bath to an internal temperature below 10°C. To the flask kept at an internal temperature of 5-10°C, 9.8 g (0.1 mol) of conc. sulfuric acid was added dropwise over 30 minutes, and stirring was continued at the temperature for 30 minutes. Subsequently, to the flask kept at an internal temperature of 5-10°C, 136.2 g (1.0 mol) of methyltrimethoxysilane was added dropwise over 30 minutes, and stirring was continued at the temperature for one hour. At an internal temperature of 5-25°C, 105.0 g (5.8 mol) of water was added dropwise over one hour. After the completion of dropwise addition, stirring was continued at 15-25°C for 3 hours. The organic layer of the reaction solution was analyzed by GC, finding that the area percent ratio of the main product to monomethoxy and monohydroxy compounds, MeSi(OSiMe3)3/[MeSi(OMe)(OSiMe3)2 + MeSi(OH)(OSiMe3)2] was 33.7. The total content of monomethoxy and monohydroxy compounds was 0.032 mol, as determined from the area percents by GC. The reaction solution was subjected to separatory operation to remove the aqueous layer. While the organic layer was kept at a temperature of 20-25°C, 14.7 g (0.15 mol) of conc. sulfuric acid was added dropwise over 15 minutes, and stirring was continued at the temperature for 45 minutes. The organic layer after reaction with sulfuric acid was analyzed by GC, finding that the area percent ratio of the main product to monomethoxy and monohydroxy compounds, MeSi(OSiMe3)3/[MeSi(OMe)(OSiMe3)2 + MeSi(OH)(OSiMe3)2] was 1010.6. The sulfuric acid layer was removed from the reaction solution, after which the organic layer was washed with water, neutralized with aqueous sodium bicarbonate, and washed with water again. On distillation of the resulting organic layer, 284.3 g (0.92 mol) of methyltris(trimethylsiloxy)silane with a purity of 99.6% was collected as a fraction having a boiling point of 120.0-120.5°C/12 kPa. The yield was 91.5%.; Example 3 A 2000-ml four necked glass flask equipped with a reflux condenser, thermometer and stirrer was purged with nitrogen. The flask was charged with 649.6 g (4.0 mol) of hexamethyldisiloxane and 128.0 g (4.0 mol) of methanol, and cooled in an ice water bath to an internal temperature below 10°C. To the flask kept at an internal temperature of 5-10°C, 19.6 g (0.2 mol) of conc. sulfuric acid was added dropwise over 30 minutes, and stirring was continued at the temperature for 30 minutes. Subsequently, to the flask kept at an internal temperature of 5-10°C, 283.5 g (2.0 mol of pure methyltrimethoxysilane) of unpurified methyltrimethoxysilane with a purity of 96.1%, which was synthesized by de-hydrochlorination reaction of methyltrichlorosilane with methanol, was added dropwise over 45 minutes, and stirring was continued at the temperature for one hour. At an internal temperature of 5-25°C, 209.9 g (11.7 mol) of water was added dropwise over one hour. After the completion of dropwise addition, stirring was continued at 15-25°C for 3 hours. The organic layer of the reaction solution was analyzed by GC, finding that the area percent ratio of the main product to monomethoxy and monohydroxy compounds, MeSi(OSiMe3)3/[MeSi(OMe)(OSiMe3)2 + MeSi(OH)(OSiMe3)2] was 34.0. The total content of monomethoxy and monohydroxy compounds was 0.066 mol, as determined from the area percents by GC. The reaction solution was subjected to separatory operation to remove the aqueous layer. While the organic layer was kept at a temperature of 20-25°C, 39.2 g (0.4 mol) of conc. sulfuric acid was added dropwise over 15 minutes, and stirring was continued at the temperature for one hour. The organic layer after reaction with sulfuric acid was analyzed by GC, finding that the area percent ratio of the main product to monomethoxy and monohydroxy compounds, MeSi(OSiMe3)3/[MeSi(OMe)(OSiMe3)2 + MeSi(OH) (OSiMe3)2] was 917.1. The sulfuric acid layer was removed from the reaction solution, after which the organic layer was washed with water, neutralized with aqueous sodium bicarbonate, and washed with water again. On distillation of the resulting organic layer, 577.2 g (1.86 mol) of methyltris(trimethylsiloxy)silane with a purity of 99.8% was collected as a fraction having a boiling point of 120.0-120.5°C/12 kPa. The yield was 92.9%.
References:
EP1510520,2005,A1 Location in patent:Page/Page column 10-11
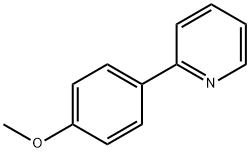
5957-90-4
79 suppliers
$39.00/1g
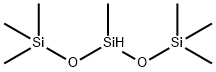
1873-88-7
287 suppliers
$5.00/5g
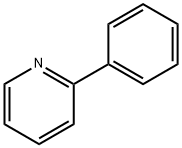
1008-89-5
275 suppliers
$7.00/5g

7671-19-4
32 suppliers
inquiry

1873-88-7
287 suppliers
$5.00/5g
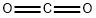
124-38-9
124 suppliers
$214.00/14L
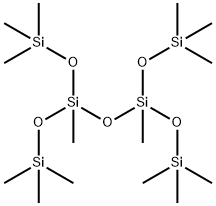
2003-92-1
8 suppliers
$853.28/5G

7671-19-4
32 suppliers
inquiry