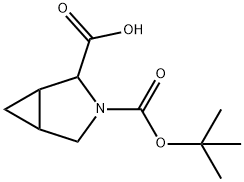
Boc-3-azabicyclo[3.1.0]hexane-2-carboxylic acid synthesis
- Product Name:Boc-3-azabicyclo[3.1.0]hexane-2-carboxylic acid
- CAS Number:953061-58-0
- Molecular formula:C11H17NO4
- Molecular Weight:227.26

24424-99-5
841 suppliers
$13.50/25G
![3-Azabicyclo[3.1.0]hexane-2-carboxylic acid](/CAS/20150408/GIF/27762-08-9.gif)
27762-08-9
14 suppliers
inquiry
![Boc-3-azabicyclo[3.1.0]hexane-2-carboxylic acid](/CAS/20140525/GIF/CB62555582.gif)
953061-58-0
14 suppliers
inquiry
Yield:-
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in dichloromethane for 22 h;
Steps:
A.8.1
A.8 Synthesis of 2-aminomethyl-3-aza-bicyclo[3.1.0]hexane-3-carboxylic acid tert-butyl ester A.8.1 Synthesis of 3-aza-bicyclo[3.1.0]hexane-2,3-dicarboxylic acid 3-tert-butyl ester;
References:
ACTELION PHARMACEUTICALS LTD WO2008/38251, 2008, A2 Location in patent:Page/Page column 94-95