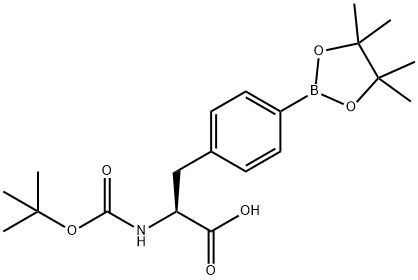
Boc-4-pinicalborane-L-phenylalanine synthesis
- Product Name:Boc-4-pinicalborane-L-phenylalanine
- CAS Number:216439-76-8
- Molecular formula:C20H30BNO6
- Molecular Weight:391.27
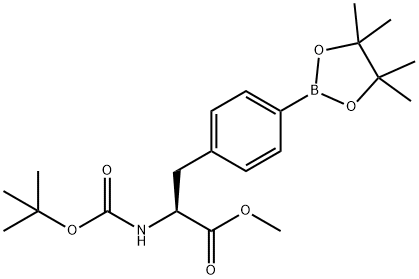
220587-29-1
37 suppliers
inquiry

216439-76-8
84 suppliers
$41.00/100mg

119771-23-2
81 suppliers
$70.00/5g
Yield: 95 %Spectr.
Reaction Conditions:
Stage #1:methyl (S)-2-((tert-butoxycarbonyl)amino)-3-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)propanoate with lithium hydroxide;water at 20; for 0.5 h;
Stage #2: with hydrogenchloride in tert-butyl methyl ether;water; pH=~ 4.5
Steps:
6.4
The above organic layer of the ester was stirred with aqueous lithium hydroxide solution (23 g in 500 mL water) at r.t. for 30 minutes. The pH of the resulting slurry was adjusted to about 10 with 6 N hydrochloric acid and filtered. The cake was washed with water (200 mL). Acetonitrile was removed from the filtrate under reduced pressure to give an aqueous slurry (950 mL, additional water was added during distillation). The slurry was filtered through a pad of 20 micron cellulose and washed with water (200 mL). The filtrate was washed with MTBE (500 mL) and rediluted with 700 mL MTBE. The mixture was acidified to pH about 4.5 with 6 N hydrochloric acid. The organic layer was washed with water (500 mL) and concentrated under reduced pressure to the acid compound as a brown oil (206 g, 95% yield based on estimated purity by NMR). The crude product can be used directly in the following step. Alternatively, the compound can be purified by crystallization from MTBE/heptane to give a white solid, which contains a small amount of the corresponding boronic acid, (S)-3-(4-boronophenyl)-2-(tert-butoxycarbonylamino)propanoic acid. MS (ESI): MH+=392.2, MNH4+=409.2, M2H+=783.4, M2NH4+=800.4. 1H NMR (CDCl3) δ 7.95 (br s, 1H), 7.76 (d, J=7.8 Hz, 2H), 7.21 (d, J=7.6 Hz, 2H), 5.03 (d, J=7.8 Hz, 1H), 4.62 (m, 1H), 3.18 (m, 2H), 1.43 (s, 9H), 1.35 (s, 12H). 13C NMR (CDCl3) δ 175.8, 155.7, 139.7, 135.4, 129.2, 84.2, 80.5, 54.5, 38.3, 28.7, 25.2.
References:
Bednarz, Mark S.;Burgoon, JR., Hugh Alfred;Iimura, Shinya;Kanamarlapudi, Ramanaiah C.;Song, Qiuling;Wu, Wenxue;Yan, Jie;Zhang, Haiming US2009/62540, 2009, A1 Location in patent:Page/Page column 8-9

220587-29-1
37 suppliers
inquiry

216439-76-8
84 suppliers
$41.00/100mg
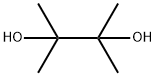
76-09-5
396 suppliers
$8.19/250mg

220587-29-1
37 suppliers
inquiry

216439-76-8
84 suppliers
$41.00/100mg
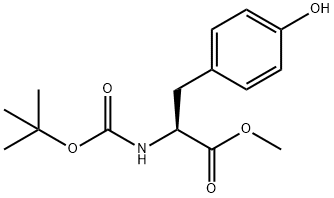
4326-36-7
369 suppliers
$6.00/5g

216439-76-8
84 suppliers
$41.00/100mg
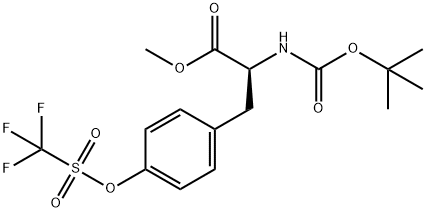
112766-18-4
32 suppliers
inquiry

216439-76-8
84 suppliers
$41.00/100mg