
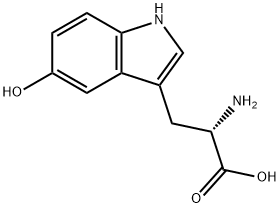
BOC-5-HYDROXY-L-TRYPTOPHAN synthesis
- Product Name:BOC-5-HYDROXY-L-TRYPTOPHAN
- CAS Number:119768-45-5
- Molecular formula:C16H20N2O5
- Molecular Weight:320.34

24424-99-5
824 suppliers
$13.50/25G
4350-09-8
431 suppliers
$5.00/100mg

119768-45-5
31 suppliers
$55.00/1g
Yield: 76%
Reaction Conditions:
with potassium carbonate in tetrahydrofuran;water at 20; for 2 h;
Steps:
5.1 Step 1. (S)-2-((tert-butoxycarbonyl)amino)-3-(5-hydroxy-1H-indol-3-yl)propanoic acid
To a solution of K2CO3 (440 mg, 3.2 mmol) in water (4 mL), L-5-hydroxy tryptophan (5-HT, 330 mg, 1.5 mmol) was added. Next, a solution of di-tert-butyl dicarbonate (392 mg, 1.8 mmol) in THF (2ml) was added to the solution and the reaction mixture was stirred at room temperature for 2 h. The reaction mixture was adjusted to pH 2-3 by addition of 1M HCl. After evaporating to remove THF, the resulting solution was extracted with ethyl acetate (10 mL x 3), the organic phase washed with brine (5 mL x 3), dried over anhydrous Na2C03, and evaporated. The residue was separated using flash chromatography (ethyl acetate as eluent) to give the desired product (76%). NMR: 12.48 (s, 1H), 10.48 (s, 1H), 8.59 (s, 1H), 7.11 (d, J = 8.0 Hz, 1H), 7.02 (d, J = 2.0 Hz, 1H), 6.93 (D, J = 8.5 Hz, 1H), 6.81 (d, J = 2.0 Hz, 1H), 6.57 (dd, Jl = 8.5 Hz, J2 = 2.0 Hz, 1H), 4.09 (m, 1H), 3.00 (dd, Jl = 28 Hz, J2 = 5Hz, 1H), 2.86 (dd, Jl = 28 Hz, J2 = 5Hz, 1H); , 1.32 (s, 9H); 13C NMR: 174.0, 155.4, 150.2, 130.6, 127.7, 124.1, 111.7, 111.2, 109.1, 102.0, 78.0, 54.3, 28.2, 26.9; LCMS found m/z: 321.3 (M+l).
References:
THE GENERAL HOSPITAL CORPORATION;CHEN, John, W.;WANG, Cuihua;KELIHER, Edmund, J. WO2018/94005, 2018, A1 Location in patent:Page/Page column 82; 83
1453-97-0
55 suppliers
$33.39/1g

119768-45-5
31 suppliers
$55.00/1g