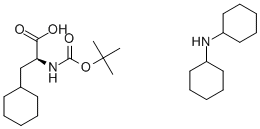
BOC-CHA-OH DCHA synthesis
- Product Name:BOC-CHA-OH DCHA
- CAS Number:37462-62-7
- Molecular formula:C26H48N2O4
- Molecular Weight:452.67

13734-34-4
549 suppliers
$5.50/5g
108-91-8
615 suppliers
$10.00/5mL

37462-62-7
44 suppliers
$24.10/1G
Yield:-
Reaction Conditions:
in diethyl ether;ethanol;rhodium;ethyl acetate;
Steps:
52 N-(t-Butoxycarbonyl)-3-cyclohexyl-L-alanine dicyclohexylamine salt
PREPARATION 52 N-(t-Butoxycarbonyl)-3-cyclohexyl-L-alanine dicyclohexylamine salt A solution of 10 g (37.7 mmole) of N-(t-butoxycarbonyl)-L-phenylalanine in 100 ml of ethanol was subjected to medium pressure hydrogenation for 14 hours in the presence of 1 g of a 5% w/w rhodium/alumina catalyst to hydrogenate the phenyl group to a cyclohexyl group. At the end of this time, the catalyst was removed by filtration, and the solvent was evaporated from the filtrate under reduced pressure. Ethyl acetate was then added to the residue, and the organic layer was washed with water and dried over anhydrous sodium sulfate. The solvent was then removed by distillation under reduced pressure. The residue was dissolved in diethyl ether, and the solution was made alkaline by the addition of cyclohexylamine and then allowed to stand at room temperature, to afford 16.6 g of the title compound as white crystals, melting at 169°-171° C., Elemental analysis: Calculated for C26 H48 N2 O4: C, 68.98%; H, 10.69%; N, 6.19%. Found: C, 68.92%; H, 10.52%; N, 6.08%.
References:
US5378690,1995,A