BOC-HSE(ME)-OH DCHA synthesis
- Product Name:BOC-HSE(ME)-OH DCHA
- CAS Number:349545-91-1
- Molecular formula:C22H42N2O5
- Molecular Weight:414.59
Yield:1007881-23-3 76%
Reaction Conditions:
Stage #1: Boc-O-methyl-L-homoserine dicyclohexylamine saltwith hydrogenchloride in isopropyl alcohol;
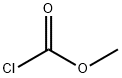
Stage #2: methyl chloroformatewith sodium carbonate;sodium hydroxide in water at 0 - 20; for 15 h;
Steps:
2b.7
2b .7 Synthesis of (S)-4-methoxy-2-(methoxycarbonylamino)butanoic acid; To i?oc-0-methyl-L-homoserine-dicyclohexylamine salt (5 g, 12.1 mmol), was added HCl in isopropanol (5-6 N, 50 mL). The mixture was stirred overnight. The volatiles were removed and the residue was dried in vacuum. To the obtained residue, water (10 mL) and NaOH (19 M, 2 mL) were added, while stirring. To this solution was added sodium carbonate (2.89 g, 27.3 mmol). The flask was cooled to 0°C in an ice- water bath. Methyl chloroformate (2.17 mL, 27.3 mmol) was added drop wise and the reaction mixture was allowed to stir for 15 hours and reach room temperature. The solvent was removed and the residue was purified by HPLC (RP Vydac Denali C 18 - ΙΟμ?ι, 250g, 5cm). Mobile phase (0.25% NH4HC03 solution in water, MeOH + CH3CN), the desired fractions were collected, and the solvent removed, yielding.N-Methoxycarbonyl-O-methyl-L-homoserine (1.77 g, 76%)
References:
WO2011/54834,2011,A1 Location in patent:Page/Page column 48-49