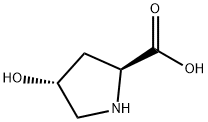
BOC-HYP-OH synthesis
- Product Name:BOC-HYP-OH
- CAS Number:331442-12-7
- Molecular formula:C10H17NO5
- Molecular Weight:231.25
Yield:-
Reaction Conditions:
with triethylamine;citric acid in acetone
Steps:
11.a (a)
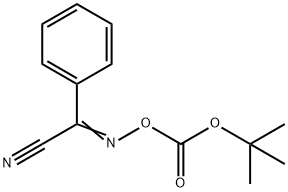
(a) (trans)-1-[(1,1-Dimethylethoxy)carbonyl]-4-hydroxy-L-proline To a solution of 20 g. (153 mmole) of (trans)-4-hydroxy-L-proline in 150 ml. of aqueous acetone is added triethylamine (32 ml., 1.5 eq.) followed by 2-(tert-butoxycarbonyloxyimino)-2-phenylacetonitrile (41.3 g., 1.1 eq.) and the resulting solution is stirred at room temperature. After 18 hours, the solution is diluted with water and extracted twice with ether. The ether fractions are discarded. The aqueous layer is acidified with 300 ml. of 0.5M citric acid and extracted four times with ethyl acetate. The organic fractions are combined and washed with water and brine. After drying (Na2 SO4), the solvent is removed at reduced pressure to give 26.55 g. of (trans)-1-[(1,1-dimethylethoxy)carbonyl]-4-hydroxy-L-proline as a pale yellow oil. Rf (silica gel, dichloromethane/methanol/acetic acid; 18:1:1)=0.64.
References:
E. R. Squibb & Sons, Inc. US4681886, 1987, A