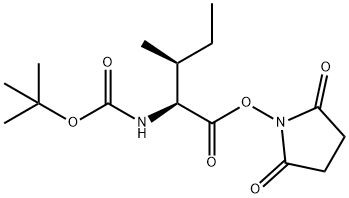
BOC-ILE-GLY-OH synthesis
- Product Name:BOC-ILE-GLY-OH
- CAS Number:16257-05-9
- Molecular formula:C13H24N2O5
- Molecular Weight:288.34

16257-04-8
5 suppliers
inquiry

16257-05-9
37 suppliers
inquiry
Yield:16257-05-9 99%
Reaction Conditions:
with lithium hydroxide monohydrate in tetrahydrofuran;methanol;water at 20; for 2 h;
Steps:
To a solution of Boc-L-Ile-Gly-OMe 20 (2.0 g, 6.6 mmol) in amixture of THF, MeOH, and H2O (1:1:1, 33 mL) was added LiOH.H2O (0.6 g, 14.3 mmol) at roomtemperature. After stirring for 2 h, the reaction mixture was quenched with 1N HCl and extractedwith EtOAc. The combined organic layer was dried over MgSO4 and concentrated in vacuo to afford1.9 g (99%) of Boc-L-Ile-Gly-OH 22 as white solid. The free acid 22 was used in the next step withoutfurther purification.
References:
Lim, Changjin [Molecules,2019,vol. 24,# 19,art. no. 3424]
![Glycine, N-[(1,1-dimethylethoxy)carbonyl]-L-isoleucyl-, ethyl ester](/CAS/20210305/GIF/56439-59-9.gif)
56439-59-9
0 suppliers
inquiry

16257-05-9
37 suppliers
inquiry
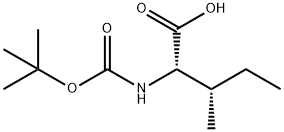
13139-16-7
335 suppliers
$11.00/5G

16257-05-9
37 suppliers
inquiry