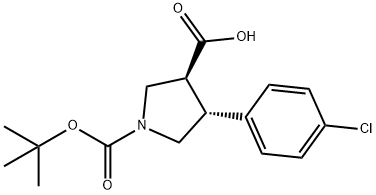
Boc-trans-DL-b-Pro-4-(4-chlorophenyl)-OH synthesis
- Product Name:Boc-trans-DL-b-Pro-4-(4-chlorophenyl)-OH
- CAS Number:851484-56-5
- Molecular formula:C16H20ClNO4
- Molecular Weight:325.79
862284-39-7
3 suppliers
inquiry

851484-56-5
17 suppliers
inquiry
Yield:851484-56-5 75%
Reaction Conditions:
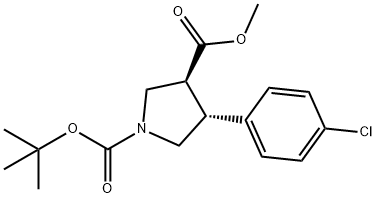
Stage #1: 1-tert-butyl 3-methyl (3S,4R)-4-(4-chlorophenyl)pyrrolidine-1,3-dicarboxylatewith lithium hydroxide in tetrahydrofuran at 20; for 24 h;
Stage #2: with hydrogenchloride in water;
Steps:
68 (3S,4R)-1-(tert-Butoxycarbonyl)-4-(4-chlorophenyl)pyrrolidine-3-carboxylic acid
To a solution of 1-tert-butyl 3-methyl (3S,4R)-4-(4-chlorophenyl)pyrrolidine-1,3-dicarboxylate, from preparation 67 (0.98 g, 2.88 mmol) in tetrahydrofuran (8 mL) was added lithium hydroxide (0.21 g, 8.64 mmol) at room temperature. The reaction mixture was stirred for 24 hours and the solvent was removed in vacuo. The crude residue was dissolved in water (8 mL) and 1 M hydrochloric acid solution (8.65 mL) was added. The suspension was extracted with dichloromethane (2*40 mL) and the organic phase was dried over magnesium sulfate, filtered and concentrated in vacuo to afforded the desired product as a white solid, 705 mg (75%). 1H NMR (400 MHz, CDCl3) δ 1.45 (s, 9H), 3.17 (m, 1H), 3.36 (m, 1H), 3.61 (m, 2H), 3.88 (m, 2H), 7.18 (d, 2H), 7.29 (d, 2H) LRMS (APCI) 226 [MH+-BOC+1] LRMS (APCI-)=324 [M-1]
References:
US2005/176772,2005,A1 Location in patent:Page/Page column 76