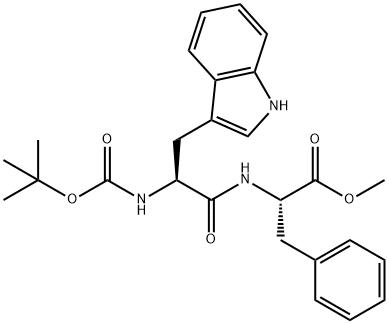
BOC-TRP-PHE-OME synthesis
- Product Name:BOC-TRP-PHE-OME
- CAS Number:72156-62-8
- Molecular formula:C26H31N3O5
- Molecular Weight:465.54
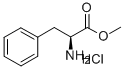
7524-50-7
412 suppliers
$5.00/5g
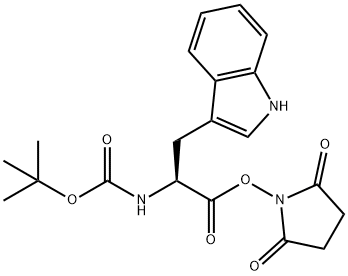
3392-11-8
80 suppliers
$12.00/250mg

72156-62-8
27 suppliers
inquiry
Yield:72156-62-8 61%
Reaction Conditions:
with sodium hydrogencarbonate in acetone at 40; for 0.166667 h;
Steps:
5 Example 5: Synthesis of Boc-Trp-(L)-Phe-OMe (CAS 72156-62-8)
Boc-(L)-Trp-OSu (5.43 g, 13.523 mmol, 1.0 eq), NaHCCE (1.36 g, 16.228 mmol, 1.2 eq), HCl H-(L)-Phe-OMe (3.21 g, 14.876 mmol, 1.1 eq) and reagent grade acetone (1.5 mL, h = 0.15 pL/mg, 1.5 eq) were introduced in a beaker and mixed up with a spatula for 30 seconds. The mixture was slowly poured into the extruder that was heated at 40°C, the speed screw being set at 150 rpm and the output valve turned towards the recirculation pipe. After 10 minutes of recirculation, the valve was opened and a white paste was recovered. After 24 h drying under reduced pressure over P2O5, the resulting white solid (m = 4.96 g, containing m = 3.44 g of pure peptide if 100% yield) was dissolved in EtOAc (150 mL) and washed once with 1M aqueous HC1 solution (50 mL) and twice with 1M aqueous NaOH solution (2 x 70 mL). The organic phase was dried over MgS04, filtered and concentrated under reduced pressure to afford the desired product as a white solid (m = 2.11 g, 61% yield, STY : 36.98 g. cm3. day 1). (0292) Details of STY calculations (0293) Estimated total mass of peptide: n(limitating reactant) x yield x M(peptide) = 13.523 x 0.61 x 465.55 = 3.85 g (0294) Reaction time: 10 minutes = 6.94 x 103 day (0295) Volume of reactor: 15 mL (0296) STY: 3.85/(15 x 6.94 x 103) = 36.98 g.cmlday 1 (0297) Details of E-factor calculations (0298) Boc-(L)-Trp-OSu: 5.43 g (0299) HCl H-(L)-Phe-OMe: 3.21 g (0300) NaHCOs: 1.36 g (0301) Acetone: 1.5 mL x 0.784 g.mL 1 = 1.18 g (0302) Total amount of reactants: 5.43 + 3.21 + 1.36 + 1.18 = 11.18 g (0303) Estimated total mass of peptide: 3.85 g (0304) E-factor = (11.18/3.85) - 1 = 1.90 (0305) (ppm) 8.21 (s, 1H), 7.66 (d, J = 7.7 Hz, 1H), 7.35 (d, J= 7.9 Hz, 1H), 7.20-7.16 (5H), 7.13 (s, 1H), 6.81 (d, J= 6.0 Hz, 2H), 6.24 (br d, J = 7.1 Hz, 1H), 5.13-5.04 (1H), 4.73 (br d, J= 6.4 Hz, 1H), 4.43 (br s, 1H), 3.61 (s, 3H), 3.41-3.28 (1H), 3.14 (dd, J= 14.5, 7.0 Hz, 1H), 2.94 (d, J= 5.8 Hz, 2H), 1.42 (s, 9H); 13C (101 MHz, CDCb) d (ppm) 171.5, 136.5, 135.8, 129.4, 128.7, 127.2, 123.5, 122.5, 120.0, 119.1, 111.4, 55.4, 53.4, 52.4, 38.1, 28.5, 25.8; MS (ESI) : m/z = 488.2 [M+Na]+ (0306) Diastereoiso meric excess was determined by HPLC analysis (Chromolith high resolution RP-l8e 50-4.6 mm, H2O/0.1%TRA in CH3CN/0.l%TFA: 0 - 100 %), Plow rate = 3 mL/min, T = 26°C, l = 214 nm, tr = 2.81 min (L,L) and tr = 2.90 min (D,L), > 99% de.
References:
WO2019/158506,2019,A1 Location in patent:Page/Page column 36; 37
2577-90-4
84 suppliers
$102.00/1g
![N-[(tert-Butoxy)carbonyl]-L-tryptophan](/CAS/GIF/13139-14-5.gif)
13139-14-5
350 suppliers
$8.00/10g

72156-62-8
27 suppliers
inquiry