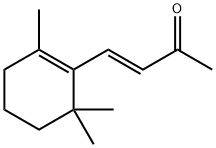
Boronal synthesis
- Product Name:Boronal
- CAS Number:3155-71-3
- Molecular formula:C14H22O
- Molecular Weight:206.32
Yield: 86.9%
Reaction Conditions:
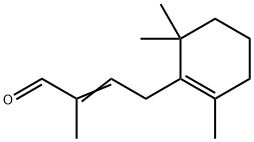
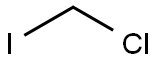
Stage #1:Chloroiodomethane;4-(2,6,6-trimethyl-1-cyclohexen-1-yl)-3-buten-2-one with lithium bromide in tetrahydrofuran at -70; for 0.05 h;
Stage #2: with trimethylsilylmethyllithium in tetrahydrofuran at 30; for 13 h;Temperature;
Steps:
1-3
Place a 200mL round-bottom flask in a low temperature reactor at -70°C, and add THF (10mL), β-ionone (1.92g), ICH2Cl (7.05g), LiBr (3.47g) to the flask in turn,After stirring for 3 min, TMSCH2Li (3.77 g) was added dropwise to the reaction solution at a rate of 0.5 mL/min. After vigorously stirring the reaction for 1h,The temperature was increased and the reaction was continued at 30°C for 12 hours, monitored by thin layer chromatography. After the reaction is complete,After adding 20 mL of saturated ammonium chloride solution and stirring for 15 minutes,The organic layer was washed with water (20 mL), saturated brine (20 mL), and dried over anhydrous sodium sulfate.Filter, remove the solvent under reduced pressure, column chromatography (eluent: dichloromethane: methanol = 10:1,Volume ratio) for further purification,The yellow liquid compound 1-(2,6,6-trimethyl-1-cyclohexenyl)-3-methyl-2-butene-4-aldehyde (1.79 g) was obtained with a yield of 86.9%.
References:
Jinan University;Yan Rian;Tan Qikun CN111704568, 2020, A Location in patent:Paragraph 0028-0045
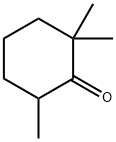
79-77-6
240 suppliers
$10.00/25g
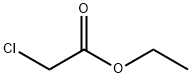
105-39-5
382 suppliers
$10.00/5g

3155-71-3
18 suppliers
inquiry

56013-05-9
0 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)