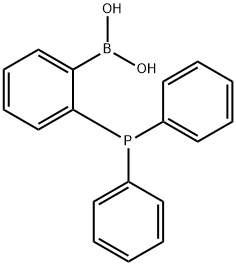
Boronic acid, B-[2-(diphenylphosphino)phenyl]- synthesis
- Product Name:Boronic acid, B-[2-(diphenylphosphino)phenyl]-
- CAS Number:1187936-76-0
- Molecular formula:C18H16BO2P
- Molecular Weight:306.1

67-56-1
757 suppliers
$9.00/25ml
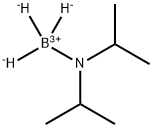
55124-35-1
23 suppliers
$60.00/100mg
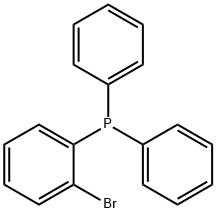
62336-24-7
118 suppliers
$24.00/250mg
![Boronic acid, B-[2-(diphenylphosphino)phenyl]-](/CAS/20200401/GIF/1187936-76-0.gif)
1187936-76-0
0 suppliers
inquiry
Yield:1187936-76-0 70%
Reaction Conditions:
Stage #1: diisopropylamine boranewith magnesium;phenylmagnesium bromide in tetrahydrofuran at 20; for 0.166667 h;
Stage #2: (2-bromophenyl)diphenylphosphane in tetrahydrofuran at 70;
Stage #3: methanolFurther stages;
Steps:
PhMgBr dehydrogenation followed by the addition under Barbier conditions (procedure B)
General procedure: To a solution in THF (4 mL) of DIPAB (863 mg, 7.5 mmol) and Mg (182 mg, 7.5 mmol) were added a PhMgBr 1M THF solution (375 μL, 375μmol) at room temperature. After 10 min, 30 mL of anhydrous THF were added followed by the arylbromide (5 mmol). The reaction mixture was cooled down to 0 °C and quenched slowly with 7 mL of MeOH. After 1h, volatile were removed under reduced pressure and the resulting solid was dissolved in 1N HCl/MeOH (7/3). After 1h at room temperature, 100 mL of AcOEt were added, the organic phase was washed with 1N HCl (30 mL) and brine (3×30 mL). Organic phases were concentrated under reduced pressure yielding a solid which was recrystallized from H2O.
References:
Marciasini, Ludovic D.;Richard, Jimmy;Cacciuttolo, Bastien;Sartori, Guillaume;Birepinte, Melodie;Chabaud, Laurent;Pinet, Sandra;Pucheault, Mathieu [Tetrahedron,2019,vol. 75,# 2,p. 164 - 171]
13061-96-6
300 suppliers
$6.00/1g
![Phosphine, diphenyl[2-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]-](/CAS/20210305/GIF/1250258-94-6.gif)
1250258-94-6
0 suppliers
inquiry

7732-18-5
475 suppliers
$13.50/100ML
![Boronic acid, B-[2-(diphenylphosphino)phenyl]-](/CAS/20200401/GIF/1187936-76-0.gif)
1187936-76-0
0 suppliers
inquiry
![Phosphine, diphenyl[2-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]-](/CAS/20210305/GIF/1250258-94-6.gif)
1250258-94-6
0 suppliers
inquiry

7732-18-5
475 suppliers
$13.50/100ML
![Boronic acid, B-[2-(diphenylphosphino)phenyl]-](/CAS/20200401/GIF/1187936-76-0.gif)
1187936-76-0
0 suppliers
inquiry

62336-24-7
118 suppliers
$24.00/250mg
![Boronic acid, B-[2-(diphenylphosphino)phenyl]-](/CAS/20200401/GIF/1187936-76-0.gif)
1187936-76-0
0 suppliers
inquiry
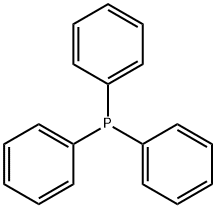
603-35-0
713 suppliers
$5.00/5G
![Boronic acid, B-[2-(diphenylphosphino)phenyl]-](/CAS/20200401/GIF/1187936-76-0.gif)
1187936-76-0
0 suppliers
inquiry